7722-84-1
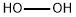
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
PRODUCT INTRODUCTION
Description
Hydrogen peroxide, (H2O2), a colourless liquid usually produced as aqueous solutions of various strengths, used principally for bleaching cotton and other textiles and wood pulp, in the manufacture of other chemicals, as a rocket propellant, and for cosmetic and medicinal purposes.
Reactions
Hydrogen peroxide decomposes into water and oxygen upon heating or in the presence of numerous substances, particularly salts of such metals as iron, copper, manganese, nickel, or chromium. It combines with many compounds to form crystalline solids useful as mild oxidizing agents; the best-known of these is sodium perborate, used in laundry detergents and chlorine-free bleach products. With certain organic compounds, hydrogen peroxide reacts to form hydroperoxides or peroxides, several of which are used to initiate polymerization reactions.
