67526-95-8
Product Name:
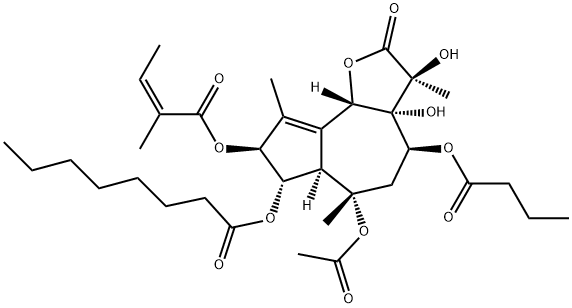
Thapsigargin
Formula:
C34H50O12
Synonyms:
;InSolution Thapsigargin - CAS 67526-95-8 - Calbiochem;Thapsigargin - CAS 67526-95-8 - Calbiochem
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 650.8 g/mol |
|---|---|
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 17 |
| Exact Mass | 650.33022703 g/mol |
| Monoisotopic Mass | 650.33022703 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Thapsigargin is an organic heterotricyclic compound that is a hexa-oxygenated 6,7-guaianolide isolated fron the roots of Thapsia garganica L., Apiaceae. A potent skin irritant, it is used in traditional medicine as a counter-irritant. Thapsigargin inhibits Ca(2+)-transporting ATPase mediated uptake of calcium ions into sarcoplasmic reticulum and is used in experimentation examining the impacts of increasing cytosolic calcium concentrations. It has a role as an EC 3.6.3.8 (Ca(2+)-transporting ATPase) inhibitor and a calcium channel blocker. It is a sesquiterpene lactone, an organic heterotricyclic compound and a butyrate ester.
