CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | 2-nitronaphthalene is a yellow crystalline solid. Mp: 76 °C; bp: 315 °C. Insoluble in water. Very soluble in ethyl alcohol and in diethyl ether. Toxic to aquatic organisms, may cause long-term adverse effects in the environment. |
|---|---|
| Boiling Point | 304 °C |
| Melting Point | 174 °F (NIOSH, 2023) |
| Solubility | Insoluble (NIOSH, 2023) |
| Vapor Density | Relative vapor density (air = 1): 5.89 |
| Vapor Pressure | 0.000258 [mmHg] |
| LogP | 2.78 |
| Ionization Potential | 8.67 eV |
| Kovats Retention Index | 1631 1631 1635 1672 1656 1631 279.2 |
| Chemical Classes | Nitrogen Compounds -> Nitros, Aromatic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H350:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 173.17 g/mol |
|---|---|
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 173.047678466 g/mol |
| Monoisotopic Mass | 173.047678466 g/mol |
| Topological Polar Surface Area | 45.8 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
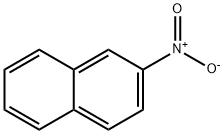
2-nitronaphthalene is a yellow crystalline solid. Mp: 76 °C; bp: 315 °C. Insoluble in water. Very soluble in ethyl alcohol and in diethyl ether. Toxic to aquatic organisms, may cause long-term adverse effects in the environment.