SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H402:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 243.22 g/mol |
|---|---|
| XLogP3 | -3.1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 243.08552052 g/mol |
| Monoisotopic Mass | 243.08552052 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 394 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
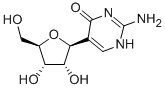
PRODUCT INTRODUCTION
description
Pseudoisocytidine is a synthetic, pyrimidine C-5 nucleoside with antineoplastic activity. Pseudoisocytidine, after conversion into pseudoisocytidine triphosphate, is incorporated into DNA and RNA eventually halting tumor cell proliferation. Compared to 5-azacytidine and cytarabine, this agent shows enhanced stability and resistance to enzymatic deamination.