53716-49-7
Product Name:
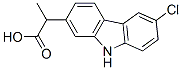
Carprofen
Formula:
C15H12ClNO2
Synonyms:
6-Chloro-α-methyl-9H-carbazole-2-acetic acid;CAR;Carprofen
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 195-199 |
| Solubility | >41.1 [ug/mL] (The mean of the results at pH 7.4) |
| LogP | 3.8 |
| Collision Cross Section | 156.1 Ų [M+H]+ [CCS Type: TW] |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 273.71 g/mol |
|---|---|
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 273.0556563 g/mol |
| Monoisotopic Mass | 273.0556563 g/mol |
| Topological Polar Surface Area | 53.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 362 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Carprofen is propanoic acid in which one of the methylene hydrogens is substituted by a 6-chloro-9H-carbazol-2-yl group. A non-steroidal anti-inflammatory drug, it is no longer used in human medicine but is still used for treatment of arthritis in elderly dogs. It has a role as a non-steroidal anti-inflammatory drug, an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor and a photosensitizing agent. It is a member of carbazoles and an organochlorine compound.
