CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | 196 |
|---|---|
| LogP | 3.23 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
COMPUTED DESCRIPTORS
| Molecular Weight | 301.72 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 301.0505709 g/mol |
| Monoisotopic Mass | 301.0505709 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 384 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
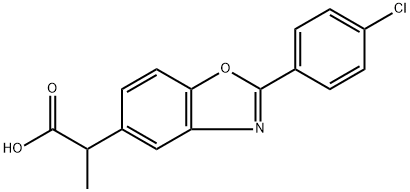
Benoxaprofen is a monocarboxylic acid that is propionic acid substituted at position 2 by a 2-(4-chlorophenyl)-1,3-benzoxazol-5-yl group. It was used as a non-steroidal anti-inflammatory drug until 1982 when it was withdrawn from the market due to adverse side-effects including liver necrosis, photosensitivity, and carcinogenicity in animals. It has a role as a non-steroidal anti-inflammatory drug, an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an antipyretic, a non-narcotic analgesic, a protein kinase C agonist, a hepatotoxic agent, an antipsoriatic and a nephrotoxin. It is a member of 1,3-benzoxazoles, a monocarboxylic acid and a member of monochlorobenzenes. It is functionally related to a propionic acid.