50-89-5
Product Name:
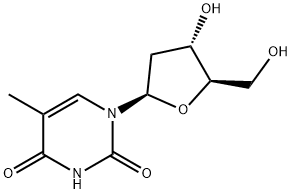
Thymidine
Formula:
C10H14N2O5
Synonyms:
Thymidine;dT;2′-Deoxythymidine;Thymidine - CAS 50-89-5 - Calbiochem;Thymine deoxyriboside
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] White odorless powder; [Alfa Aesar MSDS] |
|---|---|
| Melting Point | 186.5 °C |
| Solubility | 73.5 mg/mL |
| LogP | -0.93 |
| Stability/Shelf Life | Solution: A 1% solution of thymidine in water, kept at room temperature, is stable for at least 24 hours (HPLC). |
| Optical Rotation | (c = 1, H2O) [a]25 D = +18 ± 0.5° |
| Collision Cross Section | 161 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Chemical Classes | Biological Agents -> Nucleic Acids and Derivatives |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 242.23 g/mol |
|---|---|
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 242.09027155 g/mol |
| Monoisotopic Mass | 242.09027155 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 381 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Thymidine is a pyrimidine 2'-deoxyribonucleoside having thymine as the nucleobase. It has a role as a metabolite, a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is functionally related to a thymine. It is an enantiomer of a telbivudine.
