CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] Tetrahydrate: White solid; [Reidel-de Haen MSDS] |
|---|---|
| Color/Form | Orthorhombic crystals from anhydrous acetone or glacial acetic acid or by sublimation in vacuo. Turns pink at 230 °C ... Hot aqueous solutions are yellow and become colorless on cooling |
| Boiling Point | Sublimes |
| Melting Point | 256 °C (decomposes) |
| Solubility | Sol in benzene |
| Density | 1.07 |
| LogP | log Kow = -1.84 |
| pH | Acid to litmus |
| Dissociation Constants | pKa = 6.63 at 25 °C |
| Other Experimental Properties | Anhydrous ... Aqueous solns, after being in contact with the human skin for some time, give it a red color and a disagreeable odor. |
| Chemical Classes | Nitrogen Compounds -> Pyrimidines |
COMPUTED DESCRIPTORS
| Molecular Weight | 142.07 g/mol |
|---|---|
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 142.00145655 g/mol |
| Monoisotopic Mass | 142.00145655 g/mol |
| Topological Polar Surface Area | 92.3 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 222 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
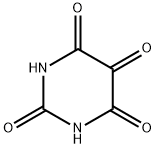
Alloxan is a member of the class of pyrimidones, the structure of which is that of perhydropyrimidine substituted at C-2, -4, -5 and -6 by oxo groups. It has a role as a hyperglycemic agent and a metabolite. It is functionally related to a barbituric acid.