38966-21-1
Product Name:
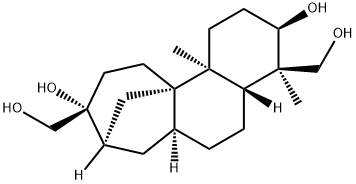
APHIDICOLIN
Formula:
C20H34O4
Synonyms:
APC;Aphidicolin - CAS 38966-21-1 - Calbiochem;ICI 69653;InSolution Aphidicolin - CAS 38966-21-1 - Calbiochem;NSC-234714
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 338.5 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 338.24570956 g/mol |
| Monoisotopic Mass | 338.24570956 g/mol |
| Topological Polar Surface Area | 80.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 524 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Aphidicolin is a tetracyclic diterpenoid that has an tetradecahydro-8,11a-methanocyclohepta[a]naphthalene skeleton with two hydroxymethyl substituents at positions 4 and 9, two methyl substituents at positions 4 and 11b and two hydroxy substituents at positions 3 and 9. An antibiotic with antiviral and antimitotical properties. Aphidicolin is a reversible inhibitor of eukaryotic nuclear DNA replication. It has a role as an antimicrobial agent, an antiviral drug, an antineoplastic agent, an EC 2.7.7.7 (DNA-directed DNA polymerase) inhibitor, a DNA synthesis inhibitor, an apoptosis inducer, a fungal metabolite, an Aspergillus metabolite and an antimitotic.
