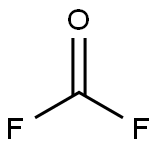
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Carbonyl fluoride appears as a colorless gas with a pungent odor. Very toxic by inhalation. Prolonged exposure of the containers to fire or heat may result in violent rupturing and rocketing. |
|---|---|
| Color/Form | COLORLESS GAS |
| Odor | Very irritating odor. |
| Boiling Point | -117 °F at 760 mmHg (USCG, 1999) |
| Melting Point | -173 °F (USCG, 1999) |
| Solubility | Reacts with water (NIOSH, 2023) |
| Density | 2.896 g/l |
| Vapor Density | 1.139 at -173.2 °F (USCG, 1999) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 55.4 atm (NIOSH, 2023) |
| Stability/Shelf Life | UNSTABLE IN PRESENCE OF WATER, INSTANTLY HYDROLYZED |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | DECOMP IN WATER AND ALC |
| Ionization Potential | 13.02 eV |
| Other Experimental Properties | MINIMUM PURITY: 97 MOLE % |
| Chemical Classes | Toxic Gases & Vapors -> Acid Halides |
COMPUTED DESCRIPTORS
| Molecular Weight | 66.007 g/mol |
|---|---|
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 65.99172094 g/mol |
| Monoisotopic Mass | 65.99172094 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 29 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Carbonyl fluoride appears as a colorless gas with a pungent odor. Very toxic by inhalation. Prolonged exposure of the containers to fire or heat may result in violent rupturing and rocketing.