3337-71-1
Product Name:
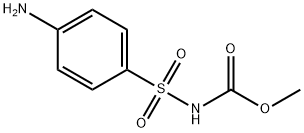
Asulam
Formula:
C8H10N2O4S
Synonyms:
Methyl N-(4-aminophenylsulfonyl)carbamate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; [Merck Index] |
|---|---|
| Color/Form | White crystals |
| Melting Point | 144.2 °C |
| Solubility | In water, 5.0X10+3 mg/L at 22 °C |
| Vapor Pressure | 0.00000144 [mmHg] |
| LogP | log Kow = -0.27 |
| Decomposition | When heated to decomposition it emits very toxic fumes of /nitrogen oxides and sulfur oxides/. |
| Dissociation Constants | pKa = 4.82 |
| Other Experimental Properties | VP: <1.3X10=2 mPa. Solubility in water: >600 g/L at 20-25 °C. Hydrolysis half-life = 63 days at pH 5, 325 °C /Asulam sodium/ |
| Chemical Classes | Pesticides -> Herbicides, Carbamate |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 230.24 g/mol |
|---|---|
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 230.03612798 g/mol |
| Monoisotopic Mass | 230.03612798 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 314 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Asulam is a carbamate ester that is methyl carbamate substituted by a (4-aminophenyl)sulfonyl group at the nitrogen atom. A dihydropteroate synthase inhibitor, it is used (normally as the corresponding sodium salt, asulam-sodium) as a herbicide, mainly for killing bracken. It has a role as an environmental contaminant, a xenobiotic, a herbicide, an agrochemical and an EC 2.5.1.15 (dihydropteroate synthase) inhibitor. It is a sulfonamide, a carbamate ester, a substituted aniline and a primary amino compound. It is a conjugate acid of an asulam(1-).
