30392-41-7
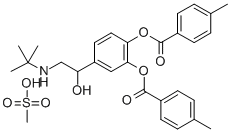
Product Name:
(tert-butyl)[beta-hydroxy-3,4-bis(p-toluoyloxy)phenethyl]ammonium methanesulphonate
Formula:
C45H64N2O5S
Inquiry
COMPUTED DESCRIPTORS
| Molecular Weight | 557.7 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 557.20833825 g/mol |
| Monoisotopic Mass | 557.20833825 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 751 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bitolterol Mesylate is the mesylate salt of bitolterol, a diester sympathomimetic amine with bronchodilator activity. As an ester prodrug, bitolterol is hydrolyzed by esterases to its active metabolite colterol (N-t-butylarterenol). Colterol selectively binds to and activates beta-2 adrenergic receptors in bronchiolar smooth muscle, thereby causing stimulation of adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cAMP). Increased intracellular cAMP levels cause relaxation of bronchial smooth muscle. This increases air flow and prevents bronchospasms and may eventually lead to an improvement of airway function.
