2691-41-0
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cyclotetramethylene tetranitramine, [wet with >= 10% water] appears as a white crystalline solid. Melting point 281 °C. Practically insoluble in water. Water slurry mitigates explosion hazard. Used in solid propellants and explosives. The primary hazard is the blast effect and not flying projectiles and fragments. |
|---|---|
| Color/Form | White crystalline solid |
| Boiling Point | Decomposition temperature = 280 °C |
| Melting Point | Melting point equals 281 °C. [CAMEO] |
| Solubility | In water, 3.34 mg/L at 20 °C, 4.46 mg/L at 25 °C |
| Density | Density of the four polymeric forms: 1.903 g/cu cm (beta form); 1.82 g/cu cm (alpha form); 1.82 g/cu cm (gamma form); 1.78 g/cu cm (delta form) |
| Vapor Density | 1.9 |
| Vapor Pressure | 0.00000002 [mmHg] |
| LogP | log Kow = 0.16 |
| Stability/Shelf Life | Stability at 300 deg K beta>alpha>gamma>delta. Beta is stable at room temperture; alpha at 115-156 °C; gamma at 156 °C; delta at /approximately/ 170-279 °C. |
| Autoignition Temperature | 234 °C |
| Decomposition | Decomposes violently at 279 °C. |
| Other Experimental Properties | Energy of formation = 353.8 kJ/kg; Enthalpy of formation = 252.8 kJ/kg |
| Chemical Classes | Other Uses -> Explosives |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P403+P235:Store in a well-ventilated place. Keep cool. |
COMPUTED DESCRIPTORS
| Molecular Weight | 296.16 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | 296.04650924 g/mol |
| Monoisotopic Mass | 296.04650924 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 322 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
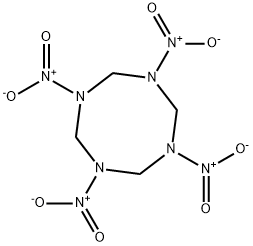
HMX (Octogen) is an acronym for High Melting eXplosive. It is also known as octogen and cyclotetramethylene-tetranitramine, as well as by other names. It is a colorless solid that dissolves slightly in water. Only a small amount of HMX (Octogen) will evaporate into the air; however, it can occur in air attached to suspended particles or dust. The taste and smell of HMX (Octogen) are not known. HMX (Octogen) does not occur naturally in the environment. It is made from other chemicals known as hexamine, ammonium nitrate, nitric acid, and acetic acid. HMX (Octogen) explodes violently at high temperatures. Because of this property, HMX (Octogen) is used in various kinds of explosives, rocket fuels, and burster chargers. A small amount of HMX (Octogen) is also formed in making cyclotrimethylene-trinitramine (RDX), another explosive similar in structure to HMX (Octogen).
