251565-85-2
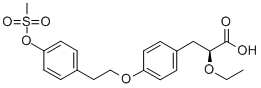
Product Name:
(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID
Formula:
C20H24O7S
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 408.5 g/mol |
|---|---|
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 408.12427427 g/mol |
| Monoisotopic Mass | 408.12427427 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 556 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tesaglitazar is a dual peroxisome proliferator-activated receptor alpha/gamma agonist which improves apolipoprotein levels in non-diabetic subjects with insulin resistance. Tesaglitazar is a proposed treatment for type 2 diabetes and has completed several phase III clinical trials, however in May 2006 AstraZeneca announced that they had discontinued further development.
