2198-61-0
Product Name:
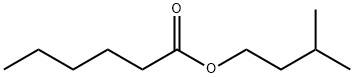
ISOAMYL HEXANOATE
Formula:
C11H22O2
Synonyms:
3-Methylbutyl hexanoate;Methylbutyl caproate, Caproic acid methylbutyl ester, Hexanoic acid methylbutyl ester
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colourless liquid with a fruity odour |
|---|---|
| Boiling Point | 225.00 to 226.00 °C. @ 760.00 mm Hg |
| Solubility | Soluble in ethanol and fixed oils, insoluble in glycerol, propylene glycol and water |
| Density | 0.858 - 0.863 |
| Refractive Index | 1.418 - 1.422 |
| Kovats Retention Index | 1253 1238 1238 1240 1238 1233 1236 1243 1227 1229 1233 1238 1232 1234 1232 1234 1234 1238 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H227:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 186.29 g/mol |
|---|---|
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 8 |
| Exact Mass | 186.161979940 g/mol |
| Monoisotopic Mass | 186.161979940 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 130 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
3-methylbutyl hexanoate is a fatty acid ester obtained by the formal condensation of the carboxy group of hexanoic acid (caproic acid) with the alcoholic hydroxy group of 3-methylbutan-1-ol (isoamylol). It has a role as a metabolite and a fragrance. It is functionally related to an isoamylol.
