CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Pale yellow crystals from isopropanol |
| Melting Point | 147-149 °C |
| Solubility | 820 mg/L |
| LogP | 2.24 |
| pH | 8-10 /Aqueous suspensions/ |
| Other Experimental Properties | /Oxamniquine/ is structurally similar to hycanthone and lucanthone. |
COMPUTED DESCRIPTORS
| Molecular Weight | 279.33 g/mol |
|---|---|
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 279.15829154 g/mol |
| Monoisotopic Mass | 279.15829154 g/mol |
| Topological Polar Surface Area | 90.1 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 332 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
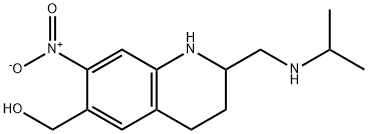
{2-[(isopropylamino)methyl]-7-nitro-1,2,3,4-tetrahydroquinolin-6-yl}methanol is a member of the class of quinolines that is 1,2,3,4-tetrahydroquinoline which is substituted at positions 2, 6, and 7 by (isopropylamino)methyl, hydroxymethyl, and nitro groups, respectively. It is a member of quinolines, a C-nitro compound, a secondary amino compound and an aromatic primary alcohol.