PRODUCT INTRODUCTION
Description
In March 2008, the European Commission granted marketing authorization for dabigatran etexilate (Pradaxa; Boehringer Ingelheim), a direct thrombin inhibitor, for the prevention of venous thromboembolic events in patients who have undergone total hip- or knee-replacement surgery.
RELATED SUPPLIERS
SRINI PHARMACEUTICALS PVT LTD
1Y
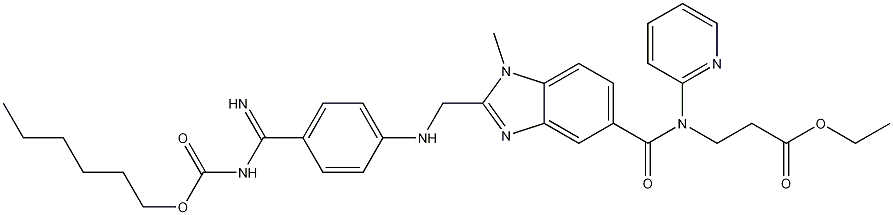
product:Ethyl 3-{[(2-{[(4-{N'-[(Hexyloxy) carbonyl] carbamimidoyl} phenyl)
amino]methyl}-l-methyl- lH-benzimidazol-5-yl)carbonyl] (2- pyridinyl)amino} propanoate 211915-06-9 98%
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Maharashtra
product:Dabigatran etexilate