CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | >254 |
|---|---|
| Solubility | soluble in methanol |
| LogP | 2.02 |
| Collision Cross Section | 194.1 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 3090 3070 3070 |
COMPUTED DESCRIPTORS
| Molecular Weight | 375.5 g/mol |
|---|---|
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 375.23220537 g/mol |
| Monoisotopic Mass | 375.23220537 g/mol |
| Topological Polar Surface Area | 66.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 506 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
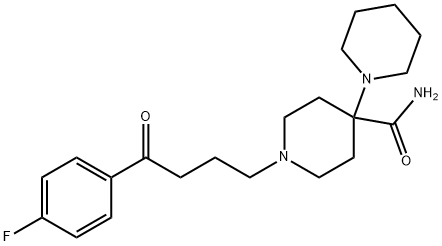
Pipamperone is a member of the class of bipiperidines that is 1,4'-bipiperidine which is substituted at the 1' and 4' positions by 4-(p-fluorophenyl)-4-oxobutyl and carboxamide groups, respectively. A first generation antipsychotic, its properties are generally similar to those of haloperidol. It has a role as a first generation antipsychotic, a serotonergic antagonist and a dopaminergic antagonist. It is a monocarboxylic acid amide, an aromatic ketone, an organofluorine compound, a member of bipiperidines and a tertiary amino compound. It is a conjugate base of a pipamperone(2+).