1642-54-2
Product Name:
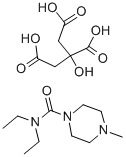
DIETHYLCARBAMAZINE CITRATE
Formula:
C16H29N3O8
Synonyms:
N,N-Diethyl-4-methyl-1-piperazinecarboxamide citrate salt;Diethylcarbamazine citrate salt
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Diethylcarbamazine citrate is a crystalline solid, scored white tablets. Used against filariasis in man and animals. (EPA, 1998) |
|---|---|
| Color/Form | White, crystalline powder |
| Odor | Odorless or slight odor |
| Taste | Bitter acid taste |
| Melting Point | 286 to 289 °F (EPA, 1998) |
| Solubility | > 75% in water @ 20 °C |
| Stability/Shelf Life | The product /as tablets/ is stable even under conditions of high temp and humidity. |
| Decomposition | When heated to decomposition it emits ... fumes of nitrogen oxides. |
| Collision Cross Section | 144.6 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | Slightly hygroscopic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
COMPUTED DESCRIPTORS
| Molecular Weight | 391.42 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 391.19546489 g/mol |
| Monoisotopic Mass | 391.19546489 g/mol |
| Topological Polar Surface Area | 159 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Diethylcarbamazine citrate is a crystalline solid, scored white tablets. Used against filariasis in man and animals. (EPA, 1998)
