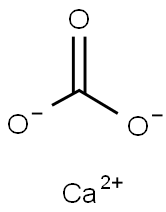
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Calcium carbonate appears as white, odorless powder or colorless crystals. Practically insoluble in water. Occurs extensive in rocks world-wide. Ground calcium carbonate (CAS: 1317-65-3) results directly from the mining of limestone. The extraction process keeps the carbonate very close to its original state of purity and delivers a finely ground product either in dry or slurry form. Precipitated calcium carbonate (CAS: 471-34-1) is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or as a by-product of the Solvay process (which is used to make sodium carbonate). Precipitated calcium carbonate is purer than ground calcium carbonate and has different (and tailorable) handling properties. |
|---|---|
| Color/Form | White hexagonal crystals or powder (Calcite); white orthrombic crystals or powder (Argonite); colorless hexagonal crystals (vaterite) |
| Odor | Odorless |
| Taste | Chalky taste |
| Boiling Point | Decomposes (NIOSH, 2023) |
| Melting Point | 1517 to 2442 °F (Decomposes) (NIOSH, 2023) |
| Solubility | 0.001 % (NIOSH, 2023) |
| Density | 2.7 to 2.95 (NIOSH, 2023) |
| Vapor Pressure | 0 mmHg (approx) (NIOSH, 2023) |
| Stability/Shelf Life | Indefinite shelflife. |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating vapors. |
| Corrosivity | Non-corrosive |
| pH | pH = 8 to 9 |
| Refractive Index | Index of Refraction: 1.7216 (300 nm); 1.6584 (589 nm); 1.6503 (750 nm) /Calcite/ |
| Other Experimental Properties | Very flowable ... surface treated with fatty acids, hydrophobic |
| Chemical Classes | Mineral Dusts -> Other Mineral Dusts |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.09 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 99.9473347 g/mol |
| Monoisotopic Mass | 99.9473347 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Calcium carbonate appears as white, odorless powder or colorless crystals. Practically insoluble in water. Occurs extensive in rocks world-wide. Ground calcium carbonate (CAS: 1317-65-3) results directly from the mining of limestone. The extraction process keeps the carbonate very close to its original state of purity and delivers a finely ground product either in dry or slurry form. Precipitated calcium carbonate (CAS: 471-34-1) is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or as a by-product of the Solvay process (which is used to make sodium carbonate). Precipitated calcium carbonate is purer than ground calcium carbonate and has different (and tailorable) handling properties.