13007-92-6
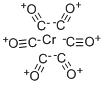
Product Name:
Chromium hexacarbonyl
Formula:
6CO.Cr
Synonyms:
Chromium(0) hexacarbonyl;Chromium carbonyl;Hexacarbonylchromium;Hexacarbonylchromium(0);Chromiumcarbonyl
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Chromium carbonyl appears as white crystalline or granular solid. Sublimes at room temperature. Burns with a luminous flame. (NTP, 1992) |
|---|---|
| Boiling Point | 410 °F at 760 mmHg (violent decomposition) (NTP, 1992) |
| Melting Point | 230 °F (decomposes) (NTP, 1992) |
| Solubility | less than 1 mg/mL at 68 °F (NTP, 1992) |
| Density | 1.77 at 64 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Pressure | 1 mmHg at 97 °F ; 760 mmHg at 304 °F (NTP, 1992) |
| Chemical Classes | Metals -> Chromium Compounds, Inorganic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 220.06 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 219.909992 g/mol |
| Monoisotopic Mass | 219.909992 g/mol |
| Topological Polar Surface Area | 6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 7 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Chromium carbonyl appears as white crystalline or granular solid. Sublimes at room temperature. Burns with a luminous flame. (NTP, 1992)
