CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | ORTHORHOMBIC BISPHENOIDAL PRISMS |
| Odor | ODORLESS |
| Taste | VERY BITTER TASTE |
| Melting Point | 176 °C |
| Solubility | PKA 6.24; FREELY SOL IN ETHANOL; INSOL IN DIETHYL ETHER /HYDROCHLORIDE/ |
| Density | 1.395 |
| Stability/Shelf Life | SUBLIMES @ 150-160 °C |
| Optical Rotation | MAX ABSORPTION (ETHANOL): 209, 291, 309-310 NM (LOG E= 4.86, 3.60, 3.69); SPECIFIC OPTICAL ROTATION: +32.7 @ 33 °C/D (WATER, 4.56%) |
| pH | NEUTRAL TO LITMUS |
| Refractive Index | INDEX OF REFRACTION: 1.5403 |
| Dissociation Constants | PK 7.8; KB 1.5X10-8 |
| Collision Cross Section | 194.3 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 3135 3168 3168 3146 3154 3170 3117 3145 3110 3170 3120 |
| Other Experimental Properties | SALTS FORMED WITH ACIDS ARE DEXTROROTATORY |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
COMPUTED DESCRIPTORS
| Molecular Weight | 413.4 g/mol |
|---|---|
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 413.14745207 g/mol |
| Monoisotopic Mass | 413.14745207 g/mol |
| Topological Polar Surface Area | 75.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 647 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
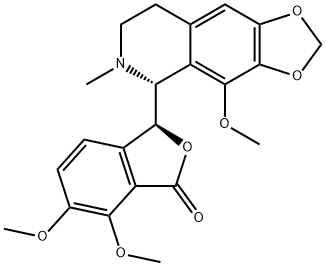
(-)-noscapine is a benzylisoquinoline alkaloid that is 1,2,3,4-tetrahydroisoquinoline which is substituted by a 4,5-dimethoxy-3-oxo-1,3-dihydro-2-benzofuran-1-yl group at position 1, a methylenedioxy group at positions 6-7 and a methoxy group at position 8. Obtained from plants of the Papaveraceae family, it lacks significant painkilling properties and is primarily used for its antitussive (cough-suppressing) effects. It has a role as an antitussive, an antineoplastic agent, an apoptosis inducer and a plant metabolite. It is a benzylisoquinoline alkaloid, a tertiary amino compound, a cyclic acetal, an isobenzofuranone, an organic heterobicyclic compound, an organic heterotricyclic compound and an aromatic ether. It is functionally related to a (-)-noscapine hemiacetal.