SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. |
COMPUTED DESCRIPTORS
| Molecular Weight | 340.5 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 340.16093457 g/mol |
| Monoisotopic Mass | 340.16093457 g/mol |
| Topological Polar Surface Area | 57.6 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 391 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
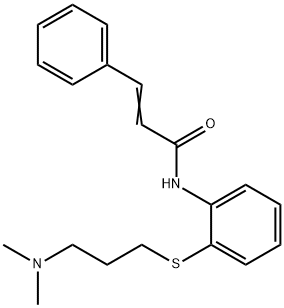
Cinanserin is an aryl sulfide that is (2E)-3-phenyl-N-(2-sulfanylphenyl)prop-2-enamide in which the hydrogen of the thiol group is substituted by a 3-(dimethylamino)propyl group. It is a 5-hydroxytryptamine receptor antagonist and an inhibitor of SARS-CoV replication. It has a role as an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor, an antiviral agent and an anticoronaviral agent. It is a tertiary amino compound, a secondary carboxamide, a member of cinnamamides and an aryl sulfide. It is a conjugate base of a cinanserin(1+).