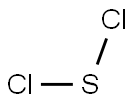CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | A fuming red to reddish-brown liquid with a chlorine odor; [HSDB] |
|---|---|
| Color/Form | Red viscous liquid |
| Odor | Pungent chlorine odor |
| Boiling Point | 59 °C |
| Melting Point | -122 °C |
| Flash Point | 245 °F (118 °C) (open cup) |
| Solubility | Slightly soluble in aliphatic hydrocarbons; very soluble in benzene and carbon tetrachloride |
| Density | 1.621 at 15 °C/15 °C |
| Vapor Density | 3.55 (Air = 1) |
| Vapor Pressure | 21.6 kPa /162 mm Hg/ at 20 °C |
| Autoignition Temperature | 453 °F (234 °C) |
| Decomposition | WHEN HEATED TO DECOMP IT EMITS ... FUMES OF /SULFUR OXIDES & HYDROGEN CHLORIDE/. |
| Viscosity | 0.548 cP at 20 °C |
| Corrosivity | Reacts violently with metals |
| Surface Tension | 33.42 mN/m at 25 °C |
| Polymerization | Aldehydes and epoxides in the presence of hydrochloric acid cause violent polymerization. Alcohol and glycols in the presence of hydrochloric acid lead to dehydration reactions. /Hydrochloric acid/ |
| Odor Threshold | Odor Threshold Low: 0.001 [ppm] |
| Refractive Index | Index of refraction: 1.567 at 20 °C |
| Other Experimental Properties | Heat of formation = -22 kJ/mol (-5.3 kcal/mol) for the gas |
| Chemical Classes | Other Classes -> Sulfur Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 102.97 g/mol |
|---|---|
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 101.9097766 g/mol |
| Monoisotopic Mass | 101.9097766 g/mol |
| Topological Polar Surface Area | 25.3 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |