41621-49-2
Product Name:
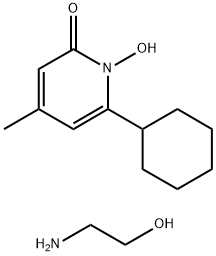
Ciclopirox ethanolamine
Formula:
C14H24N2O3
Synonyms:
6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone ethanolammonium salt
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 268.35 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 268.17869263 g/mol |
| Monoisotopic Mass | 268.17869263 g/mol |
| Topological Polar Surface Area | 86.8 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 335 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ciclopirox Olamine is the olamine salt form of ciclopirox, a synthetic, broad-spectrum antifungal agent with additional antibacterial and anti-inflammatory activities.
RELATED SUPPLIERS
Kumar Organic Products Limited
Karnataka
product:Ciclopirox Olamine USP/ EP (Kopcyclamine), 41621-49-2
