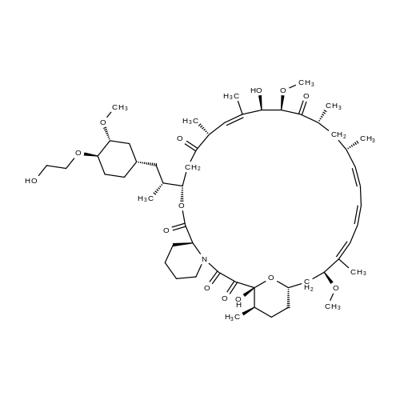
Ibrutinib
- CAS NO.:936563-96-1
- Empirical Formula: C25H24N6O2
- Molecular Weight: 440.5
- MDL number: MFCD20261150
- EINECS: 805-642-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-21 13:14:26

What is Ibrutinib?
Absorption
Ibrutinib is rapidly absorbed after oral administration and it presents a Cmax, tmax and AUC of approximately 35 ng/ml, 1-2 hour and 953 mg.h/ml respectively.
Toxicity
Ibrutinib was not showed to present a mutagenic potential in bacterial assays, nor clastogenic in chromosome aberration assays in mammalian cells or in bone marrow micronucleus assays in mice. Carcinogenicity or effects on fertility have not been determined.
Description
Ibrutinib is a cancer drug that targets B-cell malignancies such as certain leukemias and lymphomas. Its design and synthesis were reported in 2007 by Z. Pan and co-workers at Celera Genomics (South San Francisco, CA, and Rockville, MD). By that time, Pharmacyclics (Sunnyvale, CA) had acquired ibrutinib and related compounds.
Ibrutinib was approved by the US Food and Drug Administration in 2013 for treating mantle cell lymphoma and in 2014 for treating chronic lymphocytic leukemia. The lymphoma application was submitted in June 2013 and, under FDA’s breakthrough therapy program, it was approved 4 months later.
The 2013 approval was also noteworthy because?Pharmacyclics had sufficient quantities of the drug to make it commercially available?immediately. A key factor was Pharmacyclics’ long partnership with Lonza, a Switzerland-based multinational company with custom manufacturing facilities in the United States.
The Uses of Ibrutinib
Ibrutinib is an irreversible Bruton′s tyrosine kinase inhibitor that selectively blocks B cell activation.
Background
Ibrutinib is a small molecule that acts as an irreversible potent inhibitor of Burton's tyrosine kinase. It is designated as a targeted covalent drug and presented as a promising activity in B-cell malignancies in clinical trials. Ibrutinib was developed by Pharmacyclics Inc and was first approved by the FDA in November 2013 for the treatment of mantle cell lymphoma (MCL) under accelerated approval; however, in April 2023, the drug manufacturer withdrew the accelerated approvals for ibrutinib in the US.
Ibrutinib was approved by the EMA in October 2014 and by Health Canada in November 2014. It is currently approved for the treatment of various conditions, such as chronic lymphocytic leukemia (CLL), Waldenstr?m's Macroglobulinemia, and chronic graft versus host disease (cGVHD) in August 2017. Notably, ibrutinib became the first FDA-approved cGVHD treatment for children in August 2017.
Indications
Ibrutinib is indicated for the treatment of the following conditions.
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL)
Waldenstr?m's macroglobulinemia
Chronic graft-versus-host disease (cGVHD)
Mantle cell lymphoma (MCL)
Marginal zone lymphoma (MZL)
Pharmacokinetics
In vitro studies have shown an induction of CLL cell apoptosis even in presence of prosurvival factors. It has also been reported an inhibition of CLL cell survival and proliferation as well as an impaired in cell migration and a reduction in the secretion of chemokines such as CCL3 and CCL4. The latter effect has been shown to produce regression in xenograft mouse models.
Clinical studies for relapsed/refractory CLL in phase I and II showed an approximate 71% of overall response rate.. In the case of relapsed/refractory mantle cell lymphoma, approximately 70% of the tested patients presented a partial or complete response.. In clinical trials for relapsed/refractory diffuse large B-cell lymphoma, a partial response was found in between 15-20% of the patients studied; while for patients with relapsed/refractory Waldenstrom's macroglobulinemia, a partial response was observed in over 75% of the patients tested. Finally, for patients with relapsed/refractory follicular lymphoma, a partial to complete response was obtained in approximately 54% of the patients.
Metabolism
Three metabolic pathways have been identified according to the possible metabolites. These pathways are the hydroxylation of the phenyl group (M35), the opening of the piperidine with a reduction of the primary alcohol (M34) and the oxidation to a carboxylic acid and epoxidation of the ethylene followed by a hydrolysis to the formation of dihydrodiol (PCI-45227). The latter metabolite presents also 15 times lower inhibitory activity against BTK. The metabolism of ibrutinib is mainly performed by CYP3A5 and CYP3A4. and in a minor extent it is seen to be performed by CYP2D6.
Properties of Ibrutinib
| Melting point: | 153-158°C |
| Boiling point: | 715.0±60.0 °C(Predicted) |
| Density | 1.34 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO ( up to at least 25 mg/ml) |
| form | solid |
| color | White or off-white |
Safety information for Ibrutinib
Computed Descriptors for Ibrutinib
| InChIKey | XYFPWWZEPKGCCK-GOSISDBHSA-N |
| SMILES | C(N1CCC[C@@H](N2C3C(C(C4=CC=C(OC5=CC=CC=C5)C=C4)=N2)=C(N)N=CN=3)C1)(=O)C=C |
Abamectin manufacturer
Jigs Chemical ltd
Dr. Reddy's Laboratories Ltd
Bulat Pharmaceutical Pvt Ltd
Shilpa Medicare Limited (SML)
Salvavidas Pharmaceutical Pvt., Ltd.
Venkatasai Life Sciences
Basil Drugs AND Pharmaceuticals Pvt Ltd
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THF 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE)Related products of tetrahydrofuran
You may like
-
 936563-96-1 98%View Details
936563-96-1 98%View Details
936563-96-1 -
 Ibrutinib 936563-96-1 99%View Details
Ibrutinib 936563-96-1 99%View Details
936563-96-1 -
 Ibrutinib 98%View Details
Ibrutinib 98%View Details
936563-96-1 -
 Ibrutinib 99%View Details
Ibrutinib 99%View Details
936563-96-1 -
 Ibrutinib 936563-96-1 98%View Details
Ibrutinib 936563-96-1 98%View Details
936563-96-1 -
 936563-96-1 Ibrutinib 98%View Details
936563-96-1 Ibrutinib 98%View Details
936563-96-1 -
 936563-96-1 99%View Details
936563-96-1 99%View Details
936563-96-1 -
 Ibrutinib 99%View Details
Ibrutinib 99%View Details