DROXIDOPA
Synonym(s):Droxidopa;L-threo 3,4-Dihydroxyphenylserine
- CAS NO.:23651-95-8
- Empirical Formula: C9H11NO5
- Molecular Weight: 213.19
- MDL number: MFCD00799030
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-23 14:28:52
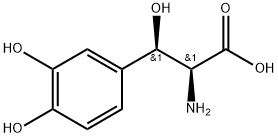
What is DROXIDOPA?
Absorption
Oral bioavailability is 90%.
Toxicity
Droxidopa has minimal toxic effects and an acute, oral LD50 of more than 5 g/kg in mice, rats, dogs, and monkeys. Side effects occur in in 0.78% of patients and include nausea, headache, increased blood pressure, hallucination, and anorexia.
Description
Droxidopa is a synthetic amino acid precursor of (-)-norepinephrine which is absorbed from the gut and metabolized to norepinephrine. In Parkinsonian patients, droxidopa added to existing levodopa/decarboxylase inhibitor therapy produces significant improvements in retropulsion, dysarthria and muscular rigidity refractory to other treatments; however, tremor was unaffected.
The Uses of DROXIDOPA
The L-threo-form Droxidopa. A synthetic amino acid precursor of Norepinephrine (N674500). Antiparkinsonian.
Background
Droxidopa is a precursor of noradrenaline that is used in the treatment of Parkinsonism. It is approved for use in Japan and is currently in trials in the U.S. The racaemic form (dl-threo-3,4-dihydroxyphenylserine) has also been used, and has been investigated in the treatment of orthostatic hypotension. There is a deficit of noradrenaline as well as of dopamine in Parkinson's disease and it has been proposed that this underlies the sudden transient freezing seen usually in advanced disease.
Though L-DOPS has been used in Japan and Southeast Asia already for some time, it is also currently in clinical trials at the phase III point in the United States (U.S.), Canada, Australia, and throughout Europe. Provided L-DOPS successfully completes clinical trials, it could be approved for the treatment of neurogenic orthostatic hypotension (NOH) as early as 2011. Additionally, phase II clinical trials for intradialytic hypotension are also underway. Chelsea Therapeutics obtained orphan drug status (ODS) for L-DOPS in the U.S. for NOH, and that of which associated with Parkinson's disease , pure autonomic failure, and multiple system atrophy, and is the pharmaceutical company developing it in that country.
Indications
For treatment of neurogenic orthostatic hypotension (NOH) associated with various disorders including Multiple System Atrophy, Familial Amyloid Polyneuropathy, hemodialysis induced hypotension and Parkinson's Disease. Also investigated for use/treatment in neurologic disorders, nephropathy, blood (blood forming organ disorders, unspecified), and dizzy/fainting spells.
Pharmacokinetics
Droxidopa is an orally active synthetic precursor of norepinephrine that increases the deficient supply of norepinephrine in patients with NOH, thereby improving orthostatic blood pressure and alleviating associated symptoms of lightheadedness, dizziness, blurred vision, and syncope through the induction of tachycardia (increased heart rate) and hypertension.
Metabolism
Droxidopa is metabolized by aromatic L-amino acid decarboxylase.
Properties of DROXIDOPA
| Melting point: | 232-235° (dec); mp 229-232° (dec) (Ohashi) |
| Boiling point: | 549.8±50.0 °C(Predicted) |
| Density | 1.608±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: ≥3mg/mL |
| form | powder |
| color | white to tan |
Safety information for DROXIDOPA
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DROXIDOPA
Abamectin manufacturer
Molsyns Research
New Products
3-N-BOC-(S)-AMINO BUTYRONITRILE 4-Piperidinopiperidine 2-Methyl-4-nitrobenzoic acid 2-(4-bromophenyl)-2-methylpropanoic acid 4-Acetyl-2-methylbenzoicacid Acetyl-meldrum's acid Ethyl-4-Pyrazole carboxylate 2,6 Di acetylpyridine 2,6-Pyridinedimethanol 5,7-Dichloro-3H-Imidazo[4,5-B]Pyridine 5-Bromo-2-Methoxy-4-Methyl-3-Nitropyridine 2-Fluoro-5-Iodopyridine 2-Fluoro-5-Methylpyridine 2-Chloro-3-Bromo-5-Amiopyridine METHYL-4-(BUTYRYLAMINO)3-METHYL-5-NITROBENZOATE TRANS-CYCLOBUTANE-1,2- DICARBOXYLIC ACID 5-Nitro indazole R-(-)-5-(2-AMINO-PROPYL)-2-METHOXY-BENZENESULFONAMIDE 1,3-cyclohexanedione 4-Aminophenaethylalchol (S)-(+)-4-BENZYL-2-OXAZOLIDINONE 3-NITRO-5-ACETYL IMINODIBENZYL 1-HYDROXY-4-METHYL6-(2,4,4-TRI METHYL PHENYL)-2-PYRIDONE MONO ETHANOL AMINE(PIROCTONE OLAMINE) 4-FLUORO PHENYL MAGNESIUM BROMIDE 1.0 M IN THFRelated products of tetrahydrofuran


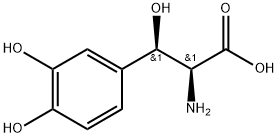
You may like
-
 23651-95-8 Droxidopa 99%View Details
23651-95-8 Droxidopa 99%View Details
23651-95-8 -
 23651-95-8 99%View Details
23651-95-8 99%View Details
23651-95-8 -
 Droxidopa 23651-95-8 98%View Details
Droxidopa 23651-95-8 98%View Details
23651-95-8 -
 23651-95-8 Droxidopa 98%View Details
23651-95-8 Droxidopa 98%View Details
23651-95-8 -
 Droxidopa 23651-95-8 98%View Details
Droxidopa 23651-95-8 98%View Details
23651-95-8 -
 23651-95-8 Droxidopa 98%View Details
23651-95-8 Droxidopa 98%View Details
23651-95-8 -
 23651-95-8 98%View Details
23651-95-8 98%View Details
23651-95-8 -
 Droxidopa Reference Standard 23651-95-8 98%View Details
Droxidopa Reference Standard 23651-95-8 98%View Details
23651-95-8