Curcumin
Synonym(s):Curcumin;Diferuloylmethane;Diferulylmethane;Natural Yellow 3;(E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
- CAS NO.:458-37-7
- Empirical Formula: C21H20O6
- Molecular Weight: 368.38
- MDL number: MFCD01868798
- EINECS: 207-280-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-31 20:01:59
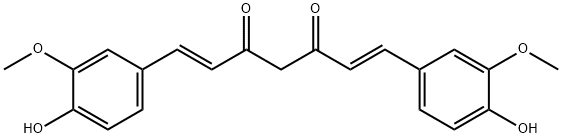
What is Curcumin?
Absorption
Curcumin displays poor absorption into the gastrointestinal tract. In a rat study, oral administration of a single dose of 2 g of curcumin resulted in a plasma concentration of less than 5 μg/mL, indicating poor absorption from the gut .
Toxicity
In an acute oral toxicity study in mouse, LD50 was >2000 mg/kg . Single oral doses of curcumin at 1-5 g/kg bw induced no toxic effects in rats . There has been no cases of overdose reported .
Description
The main source of curcumin is the root of Zingiberaceae Curcuma aromatica, rhizome of Curcuma longa (Jiang Huang), Curcuma zedoaria, and Acorus calamus. Among them, Jiang Huang contains about 3–6% curcumin. The traditional Chinese medicine, Jiang Huang, is the root tuber of perennial herbaceous plant Curcuma longa L. of family Zingiberaceae. It was firstly recorded in the “Tang materia medica” (Xin Xiu Ben Cao). It is pungent, bitter, and warm and enters the liver and spleen meridians. It activates the blood, moves qi, dredges meridians, and alleviates pain. In India and other Asian countries, Jiang Huang has more than 6000?years of application history. In Japan, Jiang Huang has a long history of health care, and the people of Okinawa Island regarded Jiang Huang as a holy tribute to the emperor. Jiang Huang mainly comes from Taiwan, Fujian, Guangdong, Guangxi, Yunnan, and Tibet of China and other regions in East Asia and Southeast Asia. It grows in warm and humid climate and sunny environment with abundant rainfall and fears cold frost, drought, and flood. At present, Chinese Pharmacopoeia only included Jiang Huang and Yu Jin which contains curcumin, while curcumin is not included.
Description
Curcumin, the bright yellow pigment in turmeric, protects the stomach from tainted foods. It inhibits the growth of?Helicobacter pylori, which has been implicated in gastric and colon cancers, but it can also reduce the effectiveness of chemotherapy.
Description
Curcumin is the major yellow pigment in turmeric and curry and has antioxidant, anti-inflammatory, and antitumor activities. It inhibits nitric oxide (NO) production (IC50 = 6 μM) and reduces inducible nitric oxide synthase (iNOS) activity in LPS-stimulated RAW 264.7 cells. Curcumin inhibits release of histamine and the inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8 from HMC-1 mast cells. In vivo, curcumin decreases serum levels of histamine and TNF-α, inhibits histopathological changes of nasal mucosa, and decreases the number of sneezes and nasal rubbing in a mouse model of ovalbumin-induced rhinitis. Curcumin (100 or 200 mg/kg) prevents ovalbumin-induced accumulation of 3-nitrotyrosine (3-NT), a marker of oxidative stress, in mouse heart. Topical administration of curcumin (1-10 μmol) reduces the number of tumors induced by phorbol 12-myristate 13-acetate (TPA; ) in mouse skin. Dietary administration of curcumin reduces the number of tongue neoplasms and preneoplastic lesions induced by 4-nitroquinoline 1-oxide (4-NQO) in rats.
Chemical properties
orange crystalline powder
Chemical properties
Several species of Curcuma exist: C. xanthorrhyza, C. domestica, C. zedoafia, C. caesia and C. amada. Although all these are aromatic plants, C. longa is the one used as a flavor ingredient. The plant is originally from southern Asia and is widespread throughout India, Malaysia, Ceylon and Japan. It is a perennial herb whose rhizome yields (like that of ginger, which it also resembles) climbing stalks with leaves only or with leaves and flowers. Reproduction occurs through the splitting of the rhizome, which is the only part used (dried rhizome as is or after previously boiling in water). Turmeric has a spicy, fresh odor reminiscent of sweet orange and ginger and a slightly pungent, bitter flavor.
Physical properties
Appearance: orange-brown crystalline powder and tastes a little bitter. It will turn into reddish brown in alkaline solution and yellow in neutral and acidic solution. It has strong stability against the reducing agent. It has excellent pigmentation which is not easy to fade. It is sensitive to light, heat, and iron ion. When PH is greater than
8, curcumin turns from yellow to red, which can be used as a pH indicator.
Solubility: insoluble in water or diethyl ether and soluble in ethanol, propylene
glycol, acetic acid, and alkali solution.
Melting point: about 183 °C.
History
Curcumin is one such agent that was described about two centuries ago as the yellow coloring matter from the rhizomes of Curcuma longa. Besides curcumin, more than 300 different components, including phenolics and terpenoids, have been identified in turmeric, but curcumin is one of the most important active components . Pure curcumin was prepared in 1842 by Vogel Jr. After 1870, the possible structure of curcumin was reported by several chemists in the subsequent decades. The chemical structure of curcumin as diferuloylmethane or 1,6-heptadiene-3,5-dione-1,7-bis (4-hydroxy-3-methoxyphenyl)-(1E, 6E) was reported by Milobedzka et?al. (1910). Lampe and Milobedzka (1913) reported the synthesis of curcumin. However, Srinivasan (1953) for the first time used chromatography to separate and quantify the components of curcumin .
Jiang Huang has been used for more than 6000 years; it is also well known for its
medicinal value and active ingredients. But it was not until the middle of the twentieth century that scientists conducted a systematic study on their pharmacological
effects. In 1949, Schraufstatter and Bernt found that curcumin has a variety of antibacterial effects against Streptococcus, Salmonella, Mucor, Mycobacterium and so
on . In the 1970s, the study also found that it has lipid-lowering, anti-inflammatory, antioxidant, and antidiabetic effects. In 1980s, it was found to have antitumor
effects. In the last 30 years, there are many reports about the clinical and pharmacological effects of curcumin.
At present, more than 65 human clinical trials have been completed, and more
than 35 clinical trials are in progress. In addition, the study of curcumin derivatives
has also become a hot topic in recent years.
The Uses of Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family (Zingiberaceae). The curcuminoids are polyphenols and are responsible for the yellow color of turmeric.
The Uses of Curcumin
A natural phenolic compound. Potent anti-tumor agent having anti-inflammatory and anti-oxidant properties. Induces apoptosis in cancer cells and inhibits phorbol ester-induced protein kinase C (PKC) activity. Reported to inhibit production of inflammatory cytokines by peripheral blood monocytes and alveolar macrophages. Potent inhibitor of EGFR tyrosine kinase and IκB kinase. Inhibits inducible nitric oxide synthase (iNOS), cycloxygenase and lipoxygenase. Easily penetrates into the cytoplasm of cells, accumulating in membranous structures such as plasma membrane, endoplasmic reticulum and nuclear envelope.
The Uses of Curcumin
antiedemic, antiinflammatory, bile stimulant; antibacterial, antifungal, lipo/cyclooxygenase inhibitor
The Uses of Curcumin
For preparing curcuma paper, pH range 8-9. In the detection of boron.
What are the applications of Application
Curcumin is a yellow-orange dye, NOS2 regulator, and inhibitor of NF-κB, 5-LO, Cox-2, COP9, and p300/CBP.
Background
Curcumin, also known as diferuloylmethane, is an active component in the golden spice turmeric (Curcuma longa) and in Curcuma xanthorrhiza oil. It is a highly pleiotropic molecule that exhibits antibacterial, anti-inflammatory, hypoglycemic, antioxidant, wound-healing, and antimicrobial activities . Due to these properties, curcumin has been investigated for the treatment and supportive care of clinical conditions including proteinuria, breast cancer, multiple myeloma, depression, and Non Small Cell Lung Cancer (NSCLC).
Despite proven efficacy against numerous experimental models, poor bioavailability due to poor absorption, rapid metabolism, and rapid systemic elimination have been shown to limit the therapeutic efficacy of curcumin . Curcumin is under investigation for the treatment and supportive care of various clinical conditions including mucositis, rectal cancer, prostate cancer, chronic schizophrenia, and Mild Cognitive Impairment (MCI) .
Indications
No approved therapeutic indications.
What are the applications of Application
Curcumin (Synthetic) is reported to suppress COX-1, COX-2, NF-κB and other inflammatory mediators
Definition
ChEBI: A beta-diketone that is methane in which two of the hydrogens are substituted by feruloyl groups. A natural dyestuff found in the root of Curcuma longa.
General Description
Orange-yellow needles.
Air & Water Reactions
Slightly soluble in hot water .
Reactivity Profile
Curcumin is sensitive to light and changes in pH. Curcumin may react with oxidizing materials.
Biological Activity
Antitumor, anti-inflammatory and antioxidant agent. Downregulates expression of reactive-oxygen-generating enzymes (cyclooxygenase, lipoxygenase, iNOS), TNF α , IL-1, IL-6, PKC, EGFR, NF- κ B, I κ B kinase and more. Upregulates expression of PPAR γ , p53, Nrf2. Also displays antimicrobial, antidiabetic neuro- and cardioprotective properties in vivo .
Biochem/physiol Actions
A natural phenolic compound. Potent anti-tumor agent having anti-inflammatory and anti-oxidant properties. Curcumin has been cited as a potential chemopreventive agent, in addition to its chemotherapeutic activity. Induces apoptosis in cancer cells and inhibits phorbol ester-induced protein kinase C (PKC) activity. Reported to inhibit production of inflammatory cytokines by peripheral blood monocytes and alveolar macrophages. Potent inhibitor of EGFR tyrosine kinase and IκB kinase. Inhibits inducible nitric oxide synthase (iNOS), cycloxygenase and lipoxygenase. Easily penetrates into the cytoplasm of cells, accumulating in membranous structures such as plasma membrane, endoplasmic reticulum and nuclear envelope.
Mechanism of action
Curcumin, the active component of turmeric (Curcuma longa), has been regarded as an anti-inflammatory and antioxidant agent . Particularly, it can scavenge reactive oxygen species, such as hydroxyl radicals, superoxide anion radicals, and nitrogen dioxide radicals. Additionally, it serves as an anti-inflammatory by down-regulating the production of pro-inflammatory cytokines (e.g., IL-1 and TNF-α) and inhibiting the activation of specific transcription factors (e.g., NF-κB and AP-1). Curcumin also demonstrates antiproliferative properties. Specifically, it inhibits UV radiation-induced skin cancer in SKH-1 hairless mice and reduces UVB-induced matrix metalloproteinase-1/3 expression in human dermal fibroblasts via MAPK-p38/JNK pathway suppression.
Pharmacokinetics
Intravenous application of 25 mg/kg bw curcumin to rats resulted in an increase in bile flow by 80 and 120% . In the rat model of inflammation, curcumin was shown to inhibit edema formation. In nude mouse that had been injected subcutaneously with prostate cancer cells, administration of curcumin caused a marked decrease in the extent of cell proliferation, a significant increase of apoptosis and micro-vessel density . Curcumin may exert choleretic effects by increasing biliary excretion of bile salts, cholesterol, and bilirubin, as well as increasing bile solubility . Curcumin inhibited arachidonic acid-induced platelet aggregation in vitro .
Pharmacology
1. Anti-fibrosis effects: curcumin has the effect of anti-fibrosis in the lung, liver, kidney, and so on. It could inhibit the release of various inflammatory factors and reduce the expression of collagen, laminin, hyaluronic acid, and other extracellular matrix content. It could also reduce the transforming growth factors such as TGF-尾 to inhibit cell proliferation .
2. Antitumor effects: the antitumor effect of curcumin is currently the most studied
pharmacological effects and attracts a lot of attention worldwide. Curcumin has
been proved to inhibit the proliferation of a variety of tumor cells through regulating a variety of transcription factors (NF-κB, AP-1, etc.), mitogen-activated
protein kinase (MAPK), growth factor receptor kinase (PDGFR, VEGFR, etc.),
and cyclooxygenase. It plays an important role in the cell cycle and further to
inhibit proliferation. Curcumin can also inhibit the migration of tumor cells by
activating caspase and inducing tumor cell apoptosis .
3. Anti-inflammatory effects: curcumin has a strong inhibitory effect on different
kinds of inflammation. The mechanism might relate to the reduction of the
expression of prostaglandins and leukotriene to decrease the release of various
inflammatory factors. The anti-inflammatory effect of curcumin is close to that
of nonsteroidal anti-inflammatory drugs and glucocorticoids, but it has higher
safety and lower side effects .
4. Antimicrobial effects: curcumin has a strong inhibitory effect on bacteria,
viruses, fungi, and parasites . Researchers believe that curcumin may play a
role in inhibiting microbial survival and reproduction by destroying microbial
cell membranes, inducing their genetic changes, and so on.
5. Hypolipidemic effect: many researchers believe that curcumin will become a
hypolipidemic drug with a good prospect. It can lower the levels of total blood
cholesterol and triglyceride levels, increase apolipoprotein A level, promote lowdensity lipoprotein (LDL) metabolism, and increase LDL excretion to reduce
LDL body content .
6. Drug metabolism: rats were treated with a single dose of refined curcumin orally,
60–65% of which was absorbed by the gastrointestinal tract. Within 5 days, 40%
of curcumin were excreted from the feces. The plasma concentration reached the
peak after 3 days. The transformation of curcumin happened in the process of
hepato-enteral circulation .
Anticancer Research
It is a yellow-colored polyphenolic compound found in turmeric and used as a foodadditive. It has antitumor effects involved in mutagenesis, cell cycle regulation,apoptosis, oncogene expression, and metastasis. Thus it regulates the initiation,promotion, and progression of disease (Hosseini and Ghorbani 2015). Its mechanismof action is diversified and convoluted. 10 μM curcumin suppresses binding of theTPA response element (TRE) by c-Jun/activator protein-1 in NIH 3 T3 cells ofmouse fibroblasts. Both protein kinase C and ornithine decarboxylase are alsoinhibited by curcumin. Inhibition of cyclooxygenase and lipoxygenase leads tosuppression of arachidonic acid cascade (Murakami et al. 1996). Curcumin is animpressive blocker of the activation of NF-κB by inhibiting IκB kinase (IKK).Curcumin also downregulates cyclin D1, suppresses the cell growth, and inducesapoptosis in prostate, breast, acute myelogenous leukemia, and multiple myelomacancer cells. It may act against psoriasis by inhibition of phosphorylase kinaseenzyme (Aggarwal and Shishodia 2004). Curcumin downregulates the TNF-inducedNF-κB-regulated gene products involved in cellular proliferation (cyclin D1, COX-2,c-myc), antiapoptosis (IAP2, IAP1, Bcl-2, XIAP, Bcl-xL, TRAF1, Bf1–1/A1,Cflip), and metastasis (MMP-9, VEGF, ICAM-1). It also suppresses the activity ofIκBα kinase, κBα degradation, IκBα phosphorylation, p65 nuclear translocation,p65 phosphorylation, and p65 acetylation (Aggarwal et al. 2008). It upregulates the expression of p53, p16, p21, EGR1 (early growth response protein1), ERK(extracellular signal-regulated kinase), JNK(c-Jun-N-terminal kinase), ElK1, Bax,and caspase 3, caspase8, and caspase9 proteins and downregulates Bcl2, mTOR,p65, Bcl-xL, AKT, EGFR, cdc2, retinoblastoma protein (Prb), c-myc, and cyclin D1proteins (Singh et al. 2016b). It can dissociate raptor from mTOR and inhibit mTORcomplex1. The inhibition of the Akt/mTOR signaling results from thedephosphorylation dependent on the calyculin A-sensitive protein phosphatase.Further, it modulating effect on AP-1 in HT-29 human colon cancer cells was foundto be a dose-dependent increase of AP-1 luciferase activity (Ravindran et al. 2009).
Curcumin is a dynamic element of turmeric, an outstanding Indian zest that isobtained from the plant Curcuma longa dried roots. Curcumin hindered PDGFR-incitedproliferation of human hepatic myofibroblasts (Zheng and Chen 2006). Theactivated mechanism by curcumin in PDGF signaling is as follows: Curcumindecreases the level of tyrosine phosphorylation of PDGFR-β and EGF-R; repressesthe action of ERK, JNK, and PI3/AKT; reduces cell growth; and induces apoptosisdose-dependently (Kunnumakkara et al. 2008). Moreover, curcumin interferes withPDGF signaling via relieving its inhibitory effect on PPARγ gene expression toreduce the cell growth; it also promotes the expression of PPARγ genes (Zhou et al.2007).
This compound is a yellow pigment produced by plants, mostly by those in theginger family (Zingiberaceae). Curcumin has enormous potential in terms of cancerprevention and treatment, and numerous studies and reviews described it as a potentantioxidant and anti-inflammatory agent (Aggarwal et al. 2003; Agrawal and Mishra2010). It inhibits biochemical activity, restraining overexpression of some signallingpathways and regulating the expression of tumour suppression genes (Cre?uet al. 2012). Temu kunci, or galangal (Boesenbergia pandurata), is a rhizome generallyused in cooking that can also be prepared to treat diarrhoea and mouth ulcers.It has been proven non-toxic to human skin fibroblast cells and offers protectiveeffects against colon cancer (Kirana et al. 2007). Turmeric (Curcuma longa) andginger (Zingiber officinale) are two plants that contain an abundance of curcuminand which have been investigated for their therapeutic properties. One piece ofresearch, for example, showed that ethanolic extract of turmeric showed anti-melanomaactivity against malignant melanomas (Danciu et al. 2015).
Clinical Use
1. Cholagogic effect could promote bile formation and secretion.
2. Hypolipidemic effect could reduce the level of cholesterol in the blood and prevent atherosclerosis.
3. Antibacterial and antiviral effect could inhibit Staphylococcus aureus and HIV.
4. Liver protection.
5. Anticancer and antitumor effect.
6. Help with the prevention of dementia.
7. Anti-inflammation and treatment of acne and dermatitis.
8. There are no reports of adverse effect of curcumin till now.
Metabolism
Initially, curcumin undergoes rapid intestinal metabolism to form curcumin glucuronide and curcumin sulfate via O-conjugation. Other metabolites formed include tetrahydrocurcumin, hexahydrocurcumin, and hexahydrocurcuminol via reduction . Curcumin may also undergo intensive second metabolism in the liver where the major metabolites were glucuronides of tetrahydrocurcumin and hexahydrocurcumin, with dihydroferulic acid and traces of ferulic acid as further metabolites . Hepatic metabolites are expected to be excreted in the bile . Certain curcumin metabolites, such as tetrahydrocurcumin, retain anti-inflammatory and antioxidant properties .
Side Effects
Dosages of up to 8 grams per day of curcumin have not been associated with serious adverse effects in humans. However, comprehensive long-term studies are needed to confirm this lack of adverse effects. Studies using high doses of curcumin have reported some mild adverse effects, including nausea, diarrhea, headache, skin rash, and yellow stool. Use of curcumin with piperine (a black pepper extract) may increase adverse reactions to curcumin, because piperine greatly increases intestinal permeability. Not all formulations of curcumin have been safety tested to the same degree.
Purification Methods
Crystallise curcumin from EtOH or acetic acid. [Beilstein 8 IV 3697.]
References
1) Zhang?et al.?(2015),?Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse; Cell Immunol.?298?88 2) Li?et al.?(2018),?Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells; Oncol. Lett.?16?6756
Properties of Curcumin
| Melting point: | 183 °C |
| Boiling point: | 418.73°C (rough estimate) |
| Density | 0.93 |
| vapor density | 13 (vs air) |
| refractive index | 1.4155-1.4175 |
| Flash point: | 208.9±23.6 °C |
| storage temp. | 2-8°C |
| solubility | ethanol: 10 mg/mL |
| form | powder |
| Colour Index | 75300 |
| pka | 8.09(at 25℃) |
| color | orange |
| Odor | Odorless |
| PH Range | Yellow (7.8) to red-brown (9.2) |
| Water Solubility | Slightly soluble (hot) |
| λmax | 430nm |
| Merck | 14,2673 |
| Solvent | Ethanol |
| Concentration | 1 mCi/ml |
| Specific Activity | 5-15 Ci/mmol |
| BRN | 2306965 |
| Stability: | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| Major Application | Cosmetics, drug-eluting stents, inhibition of formation of skin-wrinkles, treating alzheimer’s disease, skin diseases, coronary restenosis, diabetes, obesity, leukemia, neurofibromas, cancer, antimicrobial, antiviral, antiinflammatory, antiprostate cancer |
| CAS DataBase Reference | 458-37-7(CAS DataBase Reference) |
| EPA Substance Registry System | Curcumin (458-37-7) |
Safety information for Curcumin
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Curcumin
| InChIKey | VFLDPWHFBUODDF-FCXRPNKRSA-N |
Abamectin manufacturer
Standardcon Pvt. Ltd
AOS Products Pvt Ltd
AVIGYABIOSCIENCES PRIVATE LIMITED
HRV Global Life Sciences
New Products
Bromine 99.5% AR (4 x 500ml) Fehling's Solution No. B Amino Acid Kit of 23 items set Ammonium Molybdate Reagent Solution Beam's Reagent Solution Ehrlich's Reagent For detection of urobillinogen 4-Hydroxy Carbazole 4-((2-isopropoxyethoxy)methyl)phenol 2 – Methoxy – 5- Sulfamoyl Benzoic acid Amino Salicylic Acid. U.S.P. 1,2,3,4-Tetrahydrocarbazol-4-one Acetone Isobutryl oxime ester Curcuma aromatica Oil Curry leaf Extract Terminalia bellirica Extract Aloe vera extract 200x Withania somnifera (Ashwagandha Extract) Citrus bioflavonoids Extract (S)-4-(4-(5-(Aminomethyl)-2-Oxooxazolidin-3-Yl)Phenyl)Mo Ethyl N-[[2-[[[4-(Aminoiminomethyl)Phenyl]Amino]Methyl] 5-Nitrosalicylaldehyde 5-(Difluoromethoxy)-2-Mercapto-1H-Benzimidazole- IP/BP/ Cypermethric Acid Chloride Methyl Di Chloride (Mdc)Related products of tetrahydrofuran



You may like
-
 Curcumin 99%View Details
Curcumin 99%View Details -
 Curcumin 98%View Details
Curcumin 98%View Details -
 Curcumin 98% (HPLC) CAS 458-37-7View Details
Curcumin 98% (HPLC) CAS 458-37-7View Details
458-37-7 -
 Curcumin CAS 458-37-7View Details
Curcumin CAS 458-37-7View Details
458-37-7 -
 Curcumine, 99% CAS 458-37-7View Details
Curcumine, 99% CAS 458-37-7View Details
458-37-7 -
 Turmeric Extract 99%View Details
Turmeric Extract 99%View Details -
 Curcumin (Turmeric Yellow) CAS 458-37-7View Details
Curcumin (Turmeric Yellow) CAS 458-37-7View Details
458-37-7 -
 Curcumine, ≥94% (curcuminoid content), ≥80% (curcumin) CAS 458-37-7View Details
Curcumine, ≥94% (curcuminoid content), ≥80% (curcumin) CAS 458-37-7View Details
458-37-7