



WM 20-609
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:100kg
- Time:2020-01-19
Product Details
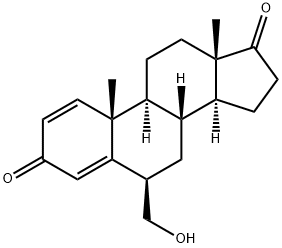
- Product NameExemestane Related Compound B (15 mg) (6-hydroxymethylandrostra-1,4-diene-3,17-dione)
- CAS No.121021-51-0
- MFC20H26O3
- MW314.41864
- AppearanceSolidWhite to Off-White
- Boiling point 492.0±45.0 °C(Predicted)
- density 1.19±0.1 g/cm3(Predicted)
▼
▲
Exemestane Related Compound B (15 mg) (6-hydroxymethylandrostra-1,4-diene-3,17-dione) Basic information
▼
▲
Synonyms:
Exemestane Related Compound B (15 mg) (6-hydroxymethylandrostra-1,4-diene-3,17-dione);6β-(HydroxyMethyl)androsta-1,4-dien-3,17-dione;6β-HydroxyMethylandrosta-1,4-diene-3,17-dione;ExeMestane Related CoMpound B;6beta-(Hydroxymethyl)androsta-1,4-dien-3,17-dione;Exemestane 6β-Hydroxymethylandrosta-1,4-diene-3,17-dione
CAS:
MF:
C20H26O3
MW:
314.41864
EINECS:
Product Categories:
Mol File:
▼
▲
Exemestane Related Compound B (15 mg) (6-hydroxymethylandrostra-1,4-diene-3,17-dione) Chemical Properties
▼
▲
▼
▲
Safety Information
▼
▲
▼
▲
Exemestane Related Compound B (15 mg) (6-hydroxymethylandrostra-1,4-diene-3,17-dione) Usage And Synthesis
▼
▲
Uses
6β-Hydroxymethylandrosta-1,4-diene-3,17-dione is a derivative of Exemestane (E957000) and a potential aromatase inhibitor.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
Recommended supplier
-
VIP3年
- GLP Pharma Standards
- 131918-64-4 Budesonide EP Impurity E/Budesonide USP Related Compound E /14,15-Dehydrobudesonide (USP) NLT 95%
- Inquiry
- 2026-01-27
-
VIP0年
- GLP Pharma Standards
- 131918-64-4 Budesonide EP Impurity E/Budesonide USP Related Compound E /14,15-Dehydrobudesonide (USP) NLT 95%
- Inquiry
- 2026-01-27
-
VIP2年
- Clickchem Research LLP
- TIZANIDINE USP RELATED COMPOUND B 173532-15-5 90 % Above
- Inquiry
- 2025-06-18
-
VIP0年
- Clickchem Research LLP
- TIZANIDINE USP RELATED COMPOUND B 173532-15-5 90 % Above
- Inquiry
- 2025-06-18
-
VIP3年
- GLP Pharma Standards
- 1132709-15-9 Bortezomib Impurity (R,R-Isomer)/Bortezomib Related compound A NLT 95%
- Inquiry
- 2026-01-27
-
VIP0年
- GLP Pharma Standards
- 1132709-15-9 Bortezomib Impurity (R,R-Isomer)/Bortezomib Related compound A NLT 95%
- Inquiry
- 2026-01-27
- Since:2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY

