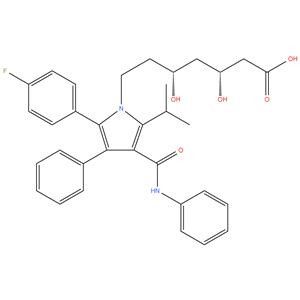
134523-00-5 98%
| Price | Get Latest Price | |||
| Packge | 1kg | 5kg | 10kg | 25kg |
- Min. Order:10kg
- Time:2024-01-22
Product Details
- Product NameAtorvastatin
- CAS No.134523-00-5
- EINECS No.806-698-0
- MFC33H35FN2O5
- MW558.65
- AppearanceSolidWhite to light yellow
- Melting point 176-178°C
- Boiling point 722.2±60.0 °C(Predicted)
- density 1.23±0.1 g/cm3(Predicted)
- storage temp. 2-8°C
Company Profile Introduction
Chempifine, a preferred Indian contract manufacturing source serving the pharma food and cosmetic industry close to two decades. To add to this we have over 10 state of art production facilities where in we undertake our production to serve our international clientele & contractual agreements. All our products meet the best international specifications as most of the time these products are manufactured against MNC or renowned companies contracts. Chempifine products have distinct advantages as we assure Top Quality, Consistency & Traceability against long term supplies contracts.
Disclaimer: Product exploration, including development, sales and offer for sales are performed where permissible by patent law. This presentation is not and should not constitute as an offer for sales in territories where it is not permitted by law.
Recommended supplier
-
VIP2年
- Clickchem Research LLP
- ATORVASTATIN ACID 134523-00-5 90 % Above
- Inquiry
- 2025-06-18
-
VIP0年
- Clickchem Research LLP
- ATORVASTATIN ACID 134523-00-5 90 % Above
- Inquiry
- 2025-06-18
-
VIP3年
- GLP Pharma Standards
- Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) 1105067-87-5 NLT 95%
- Inquiry
- 2026-01-27
-
VIP0年
- GLP Pharma Standards
- Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) 1105067-87-5 NLT 95%
- Inquiry
- 2026-01-27
-
VIP3年
- GLP Pharma Standards
- 125995-03-1 Atorvastatin Related Compound H/Atorvastatin Acid - Impurity Q (Sodium Salt) NLT 95%
- Inquiry
- 2026-01-27
-
VIP0年
- GLP Pharma Standards
- 125995-03-1 Atorvastatin Related Compound H/Atorvastatin Acid - Impurity Q (Sodium Salt) NLT 95%
- Inquiry
- 2026-01-27
-
VIP3年
- GLP Pharma Standards
- Atorvastatin Related Compound-D/Atorvastatin EP Impurity D 148146-51-4 NLT 95%
- Inquiry
- 2026-01-27
-
VIP0年
- GLP Pharma Standards
- Atorvastatin Related Compound-D/Atorvastatin EP Impurity D 148146-51-4 NLT 95%
- Inquiry
- 2026-01-27
- Since:2020-01-01
- Address: 602, Filix Tower, Opposite Asian Paints Company, Lal Bahadur Shastri Marg,Sonapur Lane, Bhandup(W),

