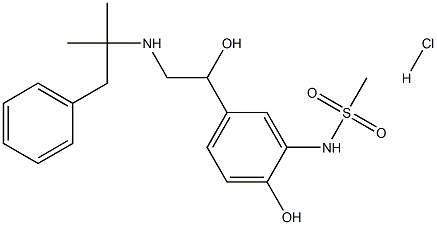
Zinterol hydrochloride
Synonym(s):N-[5-[2-[(1,1-dimethyl-2-phenylethyl)amino]-1-hydroxyethyl]-2-hydroxyphenyl]-Methanesulfonamide Hydrochloride; MJ 9184;Zinterol hydrochloride
- CAS NO.:38241-28-0
- Empirical Formula: C19H27ClN2O4S
- Molecular Weight: 414.94668
- MDL number: MFCD00867031
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:19:19
What is Zinterol hydrochloride?
Originator
Zinterol Hydrochloride,ZYF Pharm Chemical
The Uses of Zinterol hydrochloride
Zinterol Hydrochloride is an acidic salt of Zinterol (Z460000). Zinterol is a β2-adrenergic agonist. It can be used to induce smooth muscle relaxation in the lungs, gastrointestinal tract, uterus, and various blood vessels.
The Uses of Zinterol hydrochloride
Bronchodilator.
Manufacturing Process
A). 2'-Hydroxy-5'-([N-(2-methyl-1-phenyl-2-propyl)glycyl]methane-
sulfonanilide hydrobromide:
To a solution of α,α-dimethylphenthylamine (120 g, 0.8 mole) in 1.1 liter of
acetonitrile is added 5'-bromoacetyl-2'-hydroxymethanesulfonanilide (108 g,
0.35 mole) in a period of 5 minutes. The resulting solution is refluxed for 5
minutes on a steam bath and then permitted to stand for 25 minutes at room
temperature after which it is chilled and acidified with 5 N ethanolic hydrogen
bromide. The acidified mixture is concentrated in vacuum until a thick slurry is
obtained. After standing overnight at room temperature, the slurry is filtered
and the crude product triturated with 2-butanone, filtered, washed with 2-
butanone and dried to 86.3 g, (54%), MP: 217.5-221°C (dec.).
B). 2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide hydrobromide:
2'-Hydroxy-5'-([N-(2-methyl-1-phenyl-2-propyl)glycyl]methanesulfonanilide
hydrobromide (132 g, 0.29 mole is dissolved in 2 liters of hot methanol, the
methanolic solution is allowed to cool to room temperature and 13 g 10%
palladium on carbon catalyst suspended in 50 ml of water is added.
Hydrogenation of the stirred mixture is carried out under 1-3 atm. of pressure
for 17 hours during which time 0.31 mole of hydrogen is absorbed. The
catalyst is filtered and the filtrate concentrated under reduced pressure until a
thick slurry is obtained. Isoptopanpl is added tothed slurry and the mixture is
again concentrated in vacuum to remove water by azeotropic distillation.
Trituration of residual solid with 2-propanol and collection on a filter affords
100.5 g (76% yield) of desired product, MP: 194.5-195.5°C (dec.).
2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide base:
2'-Hydroxy-5'-(1-hydroxy-2-(2-methyl-1-phenyl-2-propylamino)ethyl)
methanesulfonanilide hydrobromide (47.7 g) is refluxed with 100 ml of
methanol. The material only partly dissolves. A solution of 6.5 g of potassium
hydroxide in 25 ml of methanol is then added to the suspension followed by 1
L of water. The mixture is thoroughly stirred and cooled to 5-10°C. The
precipitate is collected on a filter and washed with water until a negative test
for bromide using silver nitrate is obtained. The product is dried in an oven at
65°C, yield 36 g.
Therapeutic Function
Bronchodilator
Storage
Store at +4°C
Properties of Zinterol hydrochloride
| storage temp. | room temp |
| solubility | DMSO: ≥15mg/mL |
| form | powder |
| color | White to off-white |
Safety information for Zinterol hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zinterol hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 Zinterol hydrochloride CAS 38241-28-0View Details
Zinterol hydrochloride CAS 38241-28-0View Details
38241-28-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
