Zinc nitrate
- CAS NO.:7779-88-6
- Empirical Formula: N2O6Zn
- Molecular Weight: 189.4
- MDL number: MFCD00149889
- EINECS: 231-943-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:14
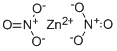
What is Zinc nitrate ?
Physical properties
The hexahydrate, Zn(NO3)2?6H2O, is a colorless and odorless crystalline solid; tetragonal structure; density 2.065 g/cm3 at 15°C; melts at 36.4°C; loses all its water of crystallization between 105 to 131°C; very soluble in water, about 184 g/100mL water at 20°C; the aqueous solution acidic, the pH of a 5% solution is about 5.1; also very soluble in alcohol
The trihydrate, Zn(NO3)2?3H2O consists of colorless needles; melts at 45.5°C; very soluble in water, 327 g/100mL at 40°C.
General Description
Zinc nitrate is a colorless crystalline solid. Noncombustible, but accelerates the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving Zinc nitrate . Zinc nitrate is used as a catalyst in the manufacture of other chemicals, in medicine, and in dyes.
Air & Water Reactions
Water soluble.
Reactivity Profile
Zinc nitrate is an oxidizing agent. Reacts violently with combustible and reducing materials. [Handling Chemicals Safely 1980. p. 967]; mixtures of Zinc nitrate s with alkyl esters may explode owing to the formation of alkyl nitrates; mixtures with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109].
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract. Contact with eyes causes irritation, which may be delayed. Contact with skin causes irritation.
Flammability and Explosibility
Not classified
Safety Profile
A powerful oxidizer. Can react violently with C, Cu, metal sulfides, organic matter, P, S. When heated to decomposition it emits toxic fumes of NOx and ZnO. See also NITRATES and ZINC COMPOUNDS.
Properties of Zinc nitrate
| Melting point: | 36 °C(lit.) |
| Density | 2.065 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| solubility | H2O: soluble |
| form | white powder |
| pka | 7.5[at 20 ℃] |
| color | white |
| Water Solubility | 1-1000000mg/L at 20℃ |
| Merck | 13,10197 |
| CAS DataBase Reference | 7779-88-6(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc nitrate (7779-88-6) |
Safety information for Zinc nitrate
Computed Descriptors for Zinc nitrate
Zinc nitrate manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Zinc Standard for IC CASView Details
Zinc Standard for IC CASView Details -
 ZINC NITRATEView Details
ZINC NITRATEView Details
7779-88-6 -
 Zinc NitrateView Details
Zinc NitrateView Details
7779-88-6 -
 crystals Zinc Nitrate (7779-88-6), Technical GradeView Details
crystals Zinc Nitrate (7779-88-6), Technical GradeView Details
7779-88-6 -
 Zinc Nitrate PowderView Details
Zinc Nitrate PowderView Details
7779-88-6 -
 Crystals Zinc Nitrate 98.99 %, Packaging Type: Drum, Packaging Size: 40 KgView Details
Crystals Zinc Nitrate 98.99 %, Packaging Type: Drum, Packaging Size: 40 KgView Details
7779-88-6 -
 Zinc Nitrate SolutionView Details
Zinc Nitrate SolutionView Details
7779-88-6 -
 Liquid H12N2O12Zn Zinc Nitrate SolutionView Details
Liquid H12N2O12Zn Zinc Nitrate SolutionView Details
10196-18-6
