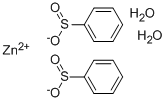
Zinc Borohydride
- CAS NO.:17611-70-0
- Empirical Formula: B2H8Zn
- Molecular Weight: 95.07552
- MDL number: MFCD20488782
- EINECS: 810-074-3
- Update Date: 2025-07-04 14:24:57
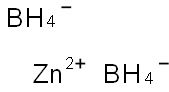
What is Zinc Borohydride?
Chemical properties
Zinc borohydride ether solution is sensitive to moisture and should be stored and used under the protection of inert gas.
The Uses of Zinc Borohydride
zinc borohydride is unique because of the better coordination ability of Zn2+, which imparts selectivity in hydridetransferring reactions. Zinc borohydride is moderately stable in ethereal solution and has many applications in organic synthesis. Zinc borohydride, as a nonconventional hydride transferring agent, has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It can be used in a range of aprotic solvents such as, THF, Et2O and DME. Several combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA, Zn(BH4)2/Me3SiCl, Zn(BH4)2/ TFA/DME, Zn(BH4 ) 2 /H2O, 1Zn(BH4 ) 2 /Al 2O3 , and Zn(BH4)2/C are interesting and have been used for different reduction purposes.
Preparation
Zinc borohydride (Zn(BH4)2) needs to be prepared by in situ reaction, anhydrous zinc chloride is reacted with sodium borohydride in anhydrous ether solvent.
Reactions
Zinc borohydride is often used as a reducing agent in organic synthesis, and its reducing ability is comparable to that of lithium aluminum hydride, and its selectivity is good. It can selectively reduce aldehydes, ketones, carboxylic acids and their derivatives, imines, nitriles, epoxy compounds, alkenes (alkynes), etc., and has good stereoselectivity for chiral molecules. Because the ether solution of zinc borohydride is neutral, it is very suitable for substrates containing base-sensitive functional groups such as cyano, ester, γ-lactone, etc. In addition, when saturated ketones and α,β-unsaturated ketones coexist, the reduction of saturated ketones can be preferentially achieved selectively.
Reduction Reaction Zinc Borohydride
Safety information for Zinc Borohydride
Zinc Borohydride manufacturer
Sainor Laboratories Pvt Ltd Unit III
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Zinc Borohydride 1.0M in THF 1.0MView Details
Zinc Borohydride 1.0M in THF 1.0MView Details
17611-70-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
