Zilucoplan
- CAS NO.:1841136-73-9
- Empirical Formula: (C2H4O)nC126H186N24O32
- Molecular Weight: 0
- Update Date: 2025-04-18 09:53:12
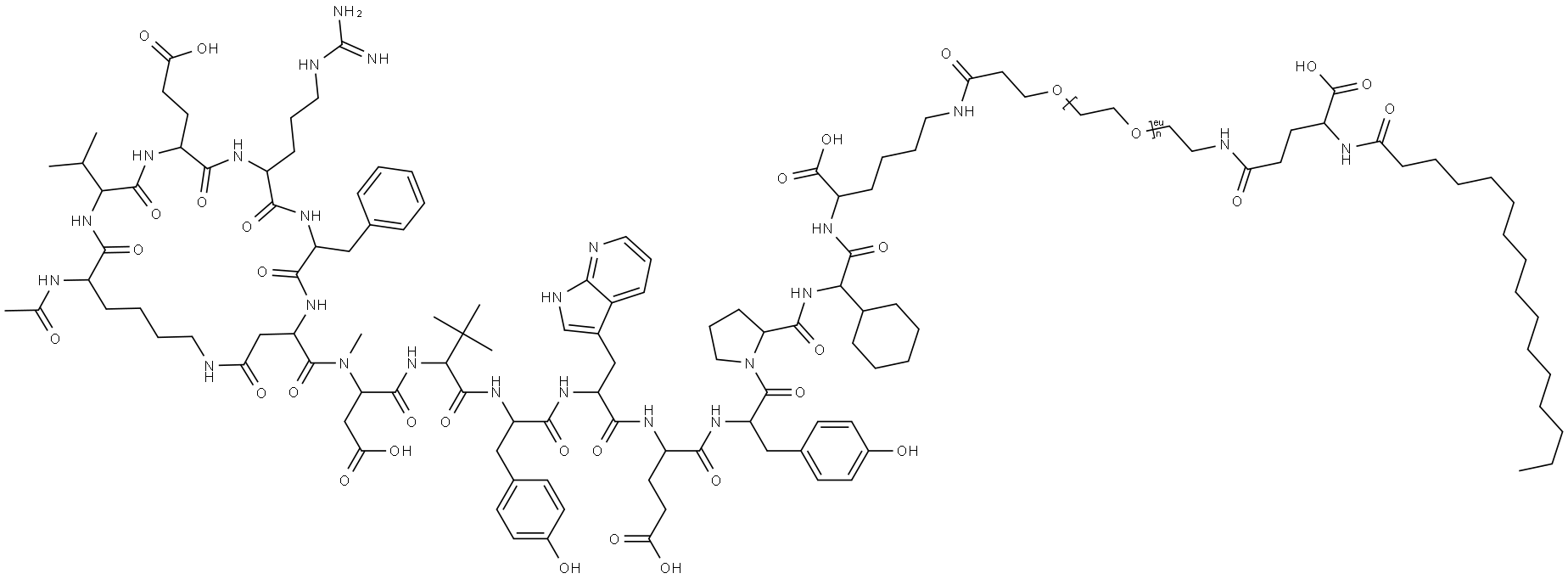
What is Zilucoplan?
Absorption
Following single and multiple daily subcutaneous administration of 0.3 mg/kg zilucoplan, time to reach peak plasma concentrations (Tmax) ranged from three to six hours. Following daily subcutaneous dosing of 0.3 mg/kg zilucoplan for 14 days in healthy subjects, both the peak plasma concentration and exposure (AUCtau) increased by approximately 3-fold.
After daily repeated subcutaneous administration of 0.3 mg/kg zilucoplan, plasma concentrations of zilucoplan were consistent, with steady state trough concentrations being reached by four weeks of treatment with zilucoplan through 12 weeks.
Toxicity
There is no information available regarding the acute toxicity (LD50) or overdose of zilucoplan.
Indications
Zilucoplan is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody-positive.
Background
Zilucoplan is a 15 amino-acid, synthetic macrocyclic peptide. It is a complement inhibitor that works to prevent the activation of C5, which is a complement protein involved in the innate immune system to initiate inflammatory responses. On October 17, 2023, zilucoplan gained its first FDA approval for the treatment of generalized myasthenia gravis.
Pharmacokinetics
In clinical trials consisting of anti-AChR antibody-positive adults with gMG, zilucoplan improved the signs and symptoms of myasthenia gravis, including muscle weakness. In vitro, it blocked the activation of C5 clinical variants. Zilucoplan causes complement inhibition in a dose-dependent manner: In clinical trials, complement inhibition of 97.5% by zilucoplan was observed by the end of the first week and persisted throughout the 12-week treatment period.
Metabolism
Zilucoplan is expected to be degraded into small peptides and amino acids via catabolic pathways. RA103488 and RA102758 are two major metabolites detected in plasma. RA103488 is formed by CYP4F2-mediated metabolism and has comparable pharmacological activity to its parent compound; however, since RA103488 is present at much lower concentrations compared to zilucoplan, its contribution to the pharmacological action of zilucoplan is expected to be low. RA102758, formed by protease-mediated degradation, is pharmacologically inactive. The AUCs of both metabolites were approximately 10% of the parent AUC.
Properties of Zilucoplan
| form | Solid |
| color | White to off-white |
Safety information for Zilucoplan
Computed Descriptors for Zilucoplan
New Products
(E)-1-Ethoxyethene-2-boronic Acid Pinacol Ester 1,3-Diethyl-1,3-Diphenylurea 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 1,3-Di Iodo Benzene Methyl 2-oxo-2,3-dihydrobenzo[d]oxazole-7-carboxylate 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 3-Hydroxy-4-nitrobromobenzene 2-Ethyl-1,4-diaminobenzene 2-Ethylhexyl 4-aminobenzoate Thio AcetamideYou may like
-
 MFCD22560726 98%View Details
MFCD22560726 98%View Details
MFCD22560726 -
 3-Hydroxy-4-nitrobromobenzene 98%View Details
3-Hydroxy-4-nitrobromobenzene 98%View Details
2768-84-0 -
 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
1234064-08-4. -
 2-Ethyl-1,4-diaminobenzene 98%View Details
2-Ethyl-1,4-diaminobenzene 98%View Details
MFCD19203885 -
 85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 -
 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details
2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details -
 Hot Sales:Thio AcetamideView Details
Hot Sales:Thio AcetamideView Details
62-55-5 -
 CosmoticsView Details
CosmoticsView Details
26218-04-2
