YC-1
Synonym(s):3-(5ʹ-Hydroxymethyl-2ʹ-furyl)-1-benzylindazole;3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl indazole;YC-1 - CAS 170632-47-0 - Calbiochem
- CAS NO.:170632-47-0
- Empirical Formula: C19H16N2O2
- Molecular Weight: 304.34
- MDL number: MFCD06407798
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-21 16:49:52
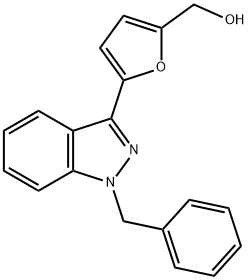
What is YC-1?
Description
YC-1 (170632-47-0) is a nitric oxide-independent activator of soluble guanylyl cyclase (sGC). Significantly elevates cGMP levels and inhibits collagen-stimulated aggregation of rabbit platelets (IC50?= 14.6 μM).1?Induces human endometrial cancer cell senescence via modulation of HIF1α activity.2?Induces degradation of HIF1α.3?Protects against glutamate-induced neuronal damage4?and β-amyloid-induced toxicity in differentiated PC12 cells5.
The Uses of YC-1
YC-1 has been used as a hypoxia-inducible factor 1α (HIF-1α) inhibitor:
- to reduce hypoxia induced Jagged1 expression in cardiomyocytes (CMs)
- to study its effect on progenitor expansion and CD34+ and side population (SP) cell phenotype and on the proliferation rate of cells with an ability to form long term colony forming units
- to study its effect on regulating sphingosine 1-phosphate (S1P) bound to albumin induced plasminogen activator inhibitor 1 (PAI-1) expression by activating Rho/ Rho-associated protein kinase (ROCK) pathway.
What are the applications of Application
YC-1 is an agent that can activate sGC independent of nitric oxide activation
Definition
ChEBI: Lificiguat is a member of the class of indazoles that is 1H-indazole which is substituted by a benzyl group at position 1 and a 5-(hydroxymethyl)-2-furyl group at position 3. It is an activator of soluble guanylate cyclase and inhibits platelet aggregation. It has a role as an antineoplastic agent, a soluble guanylate cyclase activator, an apoptosis inducer, a platelet aggregation inhibitor and a vasodilator agent. It is a member of indazoles, a member of furans and an aromatic primary alcohol.
General Description
A nitric oxide-independent, superoxide-sensitive activator of soluble guanylyl cyclase. Inhibits platelet adhesion to collagen and acts as an antithrombotic agent. Reported to reduce HIF-1α levels and xenograft growth.
Biochem/physiol Actions
YC-1 activates soluble guanylyl cyclase and prevents platelet aggregation and vascular contraction. It has a potential to treat circulation disorders. YC-1 also has an ability to inhibit hypoxia-inducible factor 1α (HIF-1α) activity in vitro. It acts as a potential antiangiogenic anticancer agent.
Storage
+4°C
References
1) Martin?et al.?(2001),?YC-1 activation of human soluble guanylyl cyclase has both heme-dependent and heme-independent components;?Proc. Natl. Acad. Sci. USA,?98?12938 2) Kato?et al.?(2006),?Induction of human endometrial cancer cell senescence through modulation of HIF-1alpha activity by EGLN1; Int. J. Cancer,?118?1144 3) Kim?et al.?(2006),?A domain responsible for HIF-1alpha degradation by YC-1, a novel anticancer agent; Int. J. Oncol.,?29?255 4) Tai?et al. (2018),?Therapeutic window for YC-1 following glutamate-induced neuronal damage and transient focal cerebral ischemia; Mol. Med. Rep.,?17?6490 5) Tsai?et al.?(2013),?The role of heat shock protein 70 in the protective effect of YC-1 on β-amyloid-induced toxicity in differentiated PC12 cells.; PLoS One,?8(7)?e69320
Properties of YC-1
| Melting point: | 110-112℃ |
| Boiling point: | 522.2±50.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: 12 mg/mL |
| pka | 13.83±0.10(Predicted) |
| form | solid |
| color | white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for YC-1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for YC-1
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![BenzaMide, N-[4-chloro-3-(trifluoroMethyl)phenyl]-2-[2-(diMethylaMino)ethoxy]-6-ethoxy-](https://img.chemicalbook.in/CAS/20150408/GIF/1311423-89-8.gif)
You may like
-
 YC-1 CAS 170632-47-0View Details
YC-1 CAS 170632-47-0View Details
170632-47-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
