XENON (129XE)
- CAS NO.:13965-99-6
- Empirical Formula: Xe
- Molecular Weight: 128.9
- MDL number: MFCD00083855
- Update Date: 2026-01-13 13:16:59
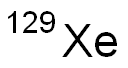
What is XENON (129XE)?
Absorption
The pharmacokinetic parameters of hyperpolarized xenon-129 are similar to those of non-polarized xenon. Xenon has low solubility; however, it is higher in fatty tissues compared to aqueous tissues and body compartments such as plasma. A study that modelled the pharmacokinetics of hyperpolarized xenon-129 suggests that 80% is the maximum concentration that can be inhaled. Hyperpolarized xenon-129 delivered through inhalation can be detected in both pulmonary and cerebral tissues.
Toxicity
Toxicity information regarding hyperpolarized Xenon-129 is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as transient hypoxia. Symptomatic and supportive measures are recommended. The mutagenic and carcinogenic potential of hyperpolarized Xenon-129 has not been evaluated.
Indications
Hyperpolarized Xenon-129 is a contrast agent indicated for use with magnetic resonance imaging (MRI) for the evaluation of lung ventilation in adults and pediatric patients aged 12 years and older.
Background
Hyperpolarized Xenon-129 (Xe-129) is a contrast agent used in the lung's clinical magnetic resonance imaging (MRI). Lung MRIs are challenging due to the low proton density of this tissue, as well as the presence of artifacts due to respiration and cardiovascular pulsation movement and air–tissue interfaces. The use of hyperpolarized noble gases makes lung MRIs feasible. They undergo a process that increases nuclear polarization up to five orders of magnitude, leading to a higher MRI signal. Helium-3 (He-3) is the one most commonly used due to its strong signal and favorable safety profile, but its use in other applications has affected its availability. Xe-129 has a lower cost, and adverse effects are normally resolved shortly after administration. In December 2022, the FDA approved the use of hyperpolarized Xe-129 for use with MRI for the evaluation of lung ventilation. Hyperpolarized Xe-129 is chemically identical to non-polarized Xe-129, and xenon is a clear, colorless, monoatomic, inert, stable, noble gas.
Definition
ChEBI: Xenon-129 atom is the stable isotope of xenon with relative atomic mass 128.904780, 26.4 atom percent natural abundance and nuclear spin 1/2. It is a xenon(0) and a xenon atom.
Pharmacokinetics
The volume of gas inhaled, the degree of xenon-129 isotopic enrichment, and the degree of hyperpolarization affect the MRI signal obtained with hyperpolarized xenon-129. When inhaled for long periods of time, xenon acts as an anesthetic at concentrations higher than 50%. Xenon has also been used in computerized tomography (CT) scans to measure cerebral blood flow.
Metabolism
As an inert gas, xenon is not metabolized under normal conditions. It does not have an effect on renal or hepatic function.
Safety information for XENON (129XE)
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1