WIN 64338 hydrochloride
- CAS NO.:163727-74-0
- Empirical Formula: C45H68ClN4OP.HCl
- Molecular Weight: 783.94
- MDL number: MFCD06804619
- Update Date: 2025-04-17 18:22:24
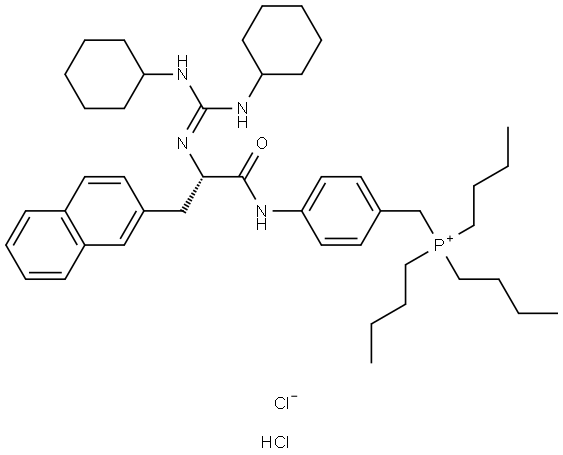
What is WIN 64338 hydrochloride?
The Uses of WIN 64338 hydrochloride
WIN 64338 Hydrochloride is a non-peptide bradykinin B2 receptor antagonist.
What are the applications of Application
WIN 64338 hydrochloride is a bradykinin B2 R Inhibitor
Biological Activity
win 64338 hydrochloride is a potent and competitive antagonist of bradykinin b2 receptor [1].bradykinin b2 receptor is a g-protein coupled receptor for bradykinin. bradykinin is an inflammatory nonapeptide and plays a critical role in edema, vasodilation, pain fiber stimulation and smooth muscle spasm.win 64338 hydrochloride is a potent and competitive bradykinin b2 receptor antagonist. in human imr-90 cells, win 64338 inhibited bradykinin binding to the bradykinin b2 receptor with ki value of 64 nm and inhibited ca2+ efflux stimulated by bradykinin with pa2 value of 7.1 in a competitive way. win 64338 inhibited guinea pig ileum contractility induced by bradykinin with pa2 value of 8.2 and also inhibited acetyicholine-induced contractility [1]. in iris sphincter isolated from rabbit, win 64338 (1-10 μm) inhibited contractile responses evoked by bradykinin with pkb value of 6.6 [2]. in guinea-pig tracheal smooth muscle cells, win 64338 inhibited inositol phosphate formation induced by bradykinin [3].in guinea-pigs, win 64338 (30 nm) significantly inhibited the increases in plasma extravasation induced by bradykinin via the release of tachykinins from the trigeminal nerve [2].
Storage
Desiccate at +4°C
References
[1]. sawutz dg, salvino jm, dolle re, et al. the nonpeptide win 64338 is a bradykinin b2 receptor antagonist. proc natl acad sci u s a, 1994, 91(11): 4693-4697.
[2]. hall jm, figini m, butt sk, et al. inhibition of bradykinin-evoked trigeminal nerve stimulation by the non-peptide bradykinin b2 receptor antagonist win 64338 in vivo and in vitro. br j pharmacol, 1995, 116(8): 3164-3168.
[3]. scherrer d, schmidlin f, lach e, et al. effect of win 64338, a b2 bradykinin receptor antagonist on guinea-pig tracheal smooth muscle cells in culture. fundam clin pharmacol, 1998, 12(2): 188-193.
Properties of WIN 64338 hydrochloride
| storage temp. | Store at -20°C |
| solubility | <58.8mg/ml in DMSO |
| form | solid |
| color | White |
Safety information for WIN 64338 hydrochloride
Computed Descriptors for WIN 64338 hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
