VX-745
- CAS NO.:209410-46-8
- Empirical Formula: C19H9Cl2F2N3OS
- Molecular Weight: 436.26
- MDL number: MFCD09834070
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-21 16:49:52
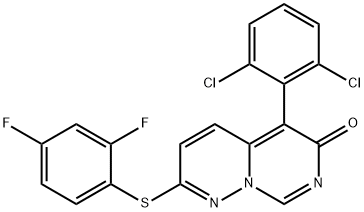
What is VX-745?
Description
VX-745 is an inhibitor of p38α MAPK (IC50 = 9 nM). It is selective for p38α over p38β MAPK (Ki = 220 nM) as well as ERK, JNK, and a panel of 50 kinases when used at a concentration of 2 μM. VX-745 inhibits LPS-induced production of IL-1β and TNF-α in isolated human peripheral blood mononuclear cells (PBMCs; IC50s = 45 and 51 nM, respectively). It reduces disease severity in a type II collagen-induced mouse model of arthritis when administered at a dose of 10 mg/kg.
Chemical properties
Pale Yellow Solid
The Uses of VX-745
VX 745 is a potent and selective inhibitor of p38α mitogen-activated protein (MAP) kinase. VX 745 is a potential anti-inflammatory agents. Studies suggest that VX 745 may be useful in the treatment of Werner syndrome.
The Uses of VX-745
VX 745 is a potent and selective inhibitor of p38α mitogen-activated protein (MAP) kinase. VX 745 is a potential anti-inflammatory agents. Studies suggest that VX 745 may be useful in the treatment of Werner syndrome.
What are the applications of Application
VX 745 is a selective and highly potent p38α and p38β inhibitor
Definition
ChEBI: A member of the class of pyrimidopyridazines that is 6H-pyrimido[1,6-b]pyridazin-6-one substituted at positions 2 and 5 by (2,4-difluorophenyl)sulfanyl and 2,6-dichlorophenyl groups respectively
Biochem/physiol Actions
VX-745 is a selective blood-brain barrier penetrant inhibitor of p38 MAPKα. VX-745 showed 20-fold selectivity for p38α over p38β and a Ki value of 220 nM. VX-745 has been shown to reduce inflammation in an arthritic (AIA) mouse model and to improve performance in aged rats.
Storage
-20°C
References
[1]. bagley, m.c., et al., microwave-assisted ullmann c-s bond formation: synthesis of the p38alpha mapk clinical candidate vx-745. j org chem, 2009. 74(21): p. 8336-42.
[2]. dent, p., et al., mapk pathways in radiation responses. oncogene, 2003. 22(37): p. 5885-96.
[3]. dominguez, c., d.a. powers, and n. tamayo, p38 map kinase inhibitors: many are made, but few are chosen. curr opin drug discov devel, 2005. 8(4): p. 421-30.
[4]. duffy, j.p., et al., the discovery of vx-745: a novel and selective p38alpha kinase inhibitor. acs med chem lett, 2011. 2(10): p. 758-63.
[5]. bagley, m.c., et al., gram-scale synthesis of the p38alpha mapk-inhibitor vx-745 for preclinical studies into werner syndrome. future med chem, 2010. 2(9): p. 1417-27.
Properties of VX-745
| Melting point: | 261-264 °C |
| Boiling point: | 578.9±60.0 °C(Predicted) |
| Density | 1.55±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | ≥21.8 mg/mL in DMSO; insoluble in H2O; ≥2.1 mg/mL in EtOH with gentle warming and ultrasonic |
| pka | -0.97±0.40(Predicted) |
| form | powder |
| color | white to beige |
Safety information for VX-745
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for VX-745
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

![1H-Pyrrole-2-carboxaMide, 4-[2-[(2-chloro-4-fluorophenyl)aMino]-5-Methyl-4-pyriMidinyl]-N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-](https://img.chemicalbook.in/CAS/GIF/896720-20-0.gif)
You may like
-
 VX745 >95% CAS 209410-46-8View Details
VX745 >95% CAS 209410-46-8View Details
209410-46-8 -
 VX-745 CAS 209410-46-8View Details
VX-745 CAS 209410-46-8View Details
209410-46-8 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
