Oteseconazole
- CAS NO.:1340593-59-0
- Empirical Formula: C23H16F7N5O2
- Molecular Weight: 527.4
- MDL number: MFCD28502267
- EINECS: 841-499-2
- Update Date: 2026-02-02 18:10:39
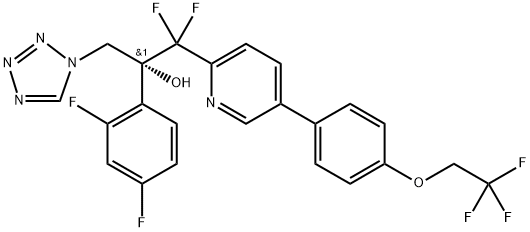
What is Oteseconazole?
Absorption
Between 20 mg and 320 mg, the AUC of oteseconazole increased relatively dose proportionally, and the Cmax increased less than dose proportionally. On average, the AUC was 64.2 h·μg/mL, and the Cmax was 2.8 μg/mL at the end of recurrent vulvovaginal candidiasis (RVVC) treatment. The tmax of oteseconazole ranged from 5 to 10 hours. Sex, race/ethnicity, and mild to moderate renal impairment do not have a significant effect on the pharmacokinetics of oteseconazole.
The bioavailability of oteseconazole is affected by high-fat, high-calorie meals. With a diet that had 800-1000 Calories and 50% fat, Cmax and AUC0-72h were 45% and 36% higher, respectively. No significant differences were detected with a low-fat, low-calorie meal. Animal models have shown that the bioavailability of oteseconazole is high. In a murine model, bioavailability was 73%. In dogs, bioavailability was 40% after fasting, and 100% in a fed state. Pre-clinical studies have shown that oteseconazole exposure in vaginal tissue is similar to plasma exposure.
Toxicity
Based on animal studies, oteseconazole may cause embryo-fetal toxicity. Additional toxicity information regarding oteseconazole is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects. In case of overdose, symptomatic and supportive measures are recommended.
In a murine carcinogenicity study, Sprague Dawley rats were administered 0.5, 1.5, or 5 mg/kg/day of oral oteseconazole. Due to high mortality, the highest dose was reduced to 3 mg/kg/day. After 77 weeks, rats receiving 5 times the maximum recommended human dose had a higher incidence of hemorrhage in the adrenals, brain, coagulating gland, ears, epididymides, head, heart, lung, nose, pancreas, pharynx, prostate, seminal vesicles, spinal cord, testes, thymus, and bladder. At 26 weeks, rats receiving 5 mg/kg/day of oteseconazole did not have a higher incidence of hemorrhage. These animal studies were performed using very high doses of oteseconazole (5 to 7 times the maximum recommended human dose), and their clinical relevance remains unclear.
Indications
Oteseconazole is an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are not of reproductive potential.
Background
Oteseconazole is an azole metalloenzyme inhibitor that targets fungal CYP51. CYP51, also known as 14α demethylase, participates in the formation of ergosterol, a compound that plays a vital role in the integrity of cell membranes. By binding and inhibiting CYP51, oteseconazole is active against most microorganisms associated with recurrent vulvovaginal candidiasis (RVVC). Oteseconazole has demonstrated activity against Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis, Candida lusitaniae and Candida dubliniensis.
Unlike previous-generation azole antifungals, oteseconazole has a high selectivity for CYP51 and little interaction with human cytochrome P450s. This is possible thanks to the tetrazole moiety in oteseconazole that increases target selectivity. In contrast with oteseconazole, other antifungals with imidazole or triazole moieties, such as ketoconazole or fluconazole, have a high number of drug-drug interactions due to their interaction with human CYPs.
The use of oteseconazole is contraindicated in females of reproductive potential due to its embryo-fetal toxicity risks. This drug was approved by the FDA on April 26, 2022.
Definition
ChEBI: Oteseconazole is an organic molecular entity.
Pharmacokinetics
Oteseconazole is a highly selective inhibitor of fungal CYP51. By targeting CYP51, oteseconazole inhibits the formation of ergosterol, a sterol required to form and maintain the integrity of fungal cell membranes. The tetrazole metal-binding group of oteseconazole increases its selectivity for fungal CYP51 and reduces off-target interactions with human cytochrome P450s. A phase 2 clinical trial that included women with vulvovaginal candidiasis reported that oteseconazole was safe and well-tolerated up to 600 mg twice daily. The exposure-response relationships and the time course of pharmacodynamic response of oteseconazole are not known. At 5-times the maximum exposures for the recommended dose, oteseconazole does not have a clinically relevant effect on QT-prolongation.
Oteseconazole is contraindicated in pregnant and lactating women and females of reproductive potential since it may cause fetal harm. The exposure window of oteseconazole is 690 days, and it precludes any mitigation measures to avoid the risk of toxicity. Several ocular abnormalities were detected in animal studies. Some of the abnormalities detected in the offspring of pregnant rats that received 7.5 mg/kg/day of oteseconazole from organogenesis to lactation were: cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage. The doses used in animal studies correspond to 3.5 times the clinical exposure detected in patients treated for recurrent vulvovaginal candidiasis (RVVC).
Metabolism
Oteseconazole does not undergo significant metabolism.
Properties of Oteseconazole
| Boiling point: | 609.2±65.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0Pa at 20-50℃ |
| storage temp. | Store at -20°C |
| solubility | DMSO: ≥ 270 mg/mL (511.96 mM) |
| form | Solid |
| pka | 10.49±0.29(Predicted) |
| color | White to off-white |
Safety information for Oteseconazole
Computed Descriptors for Oteseconazole
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
