VH298
- CAS NO.:2097381-85-4
- Empirical Formula: C27H33N5O4S
- Molecular Weight: 523.65
- MDL number: MFCD30742947
- Update Date: 2026-01-13 11:24:48
What is VH298?
Description
VH-298 is a E3 ligase ligand-linker conjugate that couples the VHL ligand and blocks the interaction between VHL and HIF-α, initiating hypoxic response.
The Uses of VH298
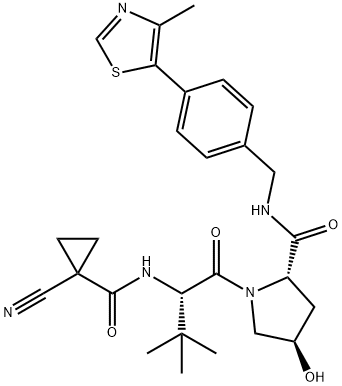
(2S,4R)-1-((S)-2-(1-Cyanocyclopropanecarboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide is a VHL inhibitors that decreases hypoxia inducible factor-α during hypoxia associated signaling in human cervical cancer, osteosarcoma HeLa and U2OS. cells.
Biological Activity
HIF-1α and hydroxy-HIF-1α levels increased in VH298-treated rFb in a time- and dose-dependent manner. Thirty micromolar VH298 could significantly increase cell proliferation, angiogenesis, and gene expression of type I collagen-α1 (Col1-α1), vascular endothelial growth factor A (VEGF-A), and insulin-like growth factor 1 (IGF-1). The VH298-treated wound had a better healing pattern, activation of HIF-1 signaling, and vascularization. It is is a potent VHL inhibitor that stabilizes HIF-α and elicits a hypoxic response via a different mechan: the the blockade of the VHL: HIF-α protein-protein interaction downstream of HIF-α hydroxylation by PHD enzymes. It engages with high affinity and specificity with VHL as its only primary cellular target, leading to selective on-target accumulation of hydroxylated HIF-α in a concentration- and time-dependent fashion in different cell lines, with subsequent upregulation of HIF-target genes at both mRNA and protein levels[1-2].
Storage
Store at -20°C
References
[1] Julianty Frost. “Potent and selective chemical probe of hypoxic signalling downstream of HIF-α hydroxylation via VHL inhibition.” Nature Communications 7 1 (2016).
[2] Shuo Qiu. “Von Hippel-Lindau (VHL) Protein Antagonist VH298 Improves Wound Healing in Streptozotocin-Induced Hyperglycaemic Rats by Activating Hypoxia-Inducible Factor- (HIF-) 1 Signalling.” Journal of Diabetes Research (2019): 1897174.
Properties of VH298
| Boiling point: | 860.8±65.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMF:30.0(Max Conc. mg/mL);57.29(Max Conc. mM) DMSO:66.42(Max Conc. mg/mL);126.84(Max Conc. mM) Ethanol:60.79(Max Conc. mg/mL);116.09(Max Conc. mM) Ethanol:PBS (pH 7.2) (1:9):0.1(Max Conc. mg/mL);0.19(Max Conc. mM) |
| form | A crystalline solid |
| pka | 12.28±0.20(Predicted) |
| color | Off-white to pink |
Safety information for VH298
Computed Descriptors for VH298
| InChIKey | NDVQUNZCNAMROD-RZUBCFFCSA-N |
| SMILES | C(NCC1=CC=C(C2SC=NC=2C)C=C1)(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@H](C(C)(C)C)NC(C1(C#N)CC1)=O |
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 VH298 CAS 2097381-85-4View Details
VH298 CAS 2097381-85-4View Details
2097381-85-4 -
 VH298 (CAS NO: 2097381-85-4)View Details
VH298 (CAS NO: 2097381-85-4)View Details
2097381-85-4 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
