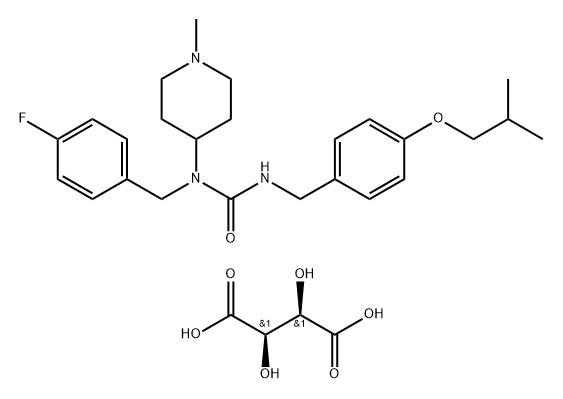
Unii-na83F1sjsr
- CAS NO.:706782-28-7
- Empirical Formula: C29H40FN3O8
- Molecular Weight: 577.65
- MDL number: MFCD09970919
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-28 14:58:11
What is Unii-na83F1sjsr?
Description
Pimavanserin, developed by San Diego-based Acadia Pharmaceuticals, is a selective and potent serotonin 2A (5-HT2A) receptor inverse agonist. The USFDA approved this once-daily drug to treat the delusions and hallucinations associated with psychosis as a function of Parkinson’s disease. Pimavanserin has a unique mechanism of action relative to other antipsychotics, behaving as a selective inverse agonist of the serotonin 5-HT2A receptor while exhibiting 40-fold selectivity over the 5-HT2C receptor and having no significant affinity or activity with the 5-HT2B or dopamine receptors.
The Uses of Unii-na83F1sjsr
Pimavanserin tartrate is a 5-HT2A inverse agonist that reverses psychosis-like behaviours and has the potential to treat various other neuropsychiatric disorders such as schizophrenia and Parkinson’s disease.
Definition
ChEBI: A tartrate salt that is the hemitartrate salt of pimavanserin. An atypical antipsychotic that is used for treatment of hallucinations and delusions associated with Parkinson's disease.
Synthesis
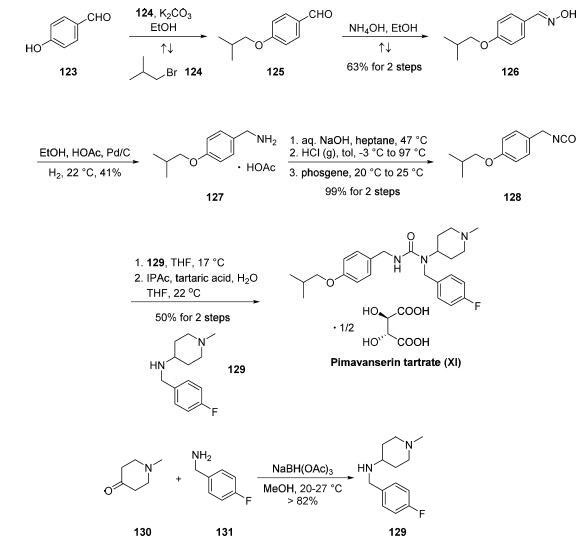
Three patent applications filed by Acadia described the process-scale synthetic approach to pimavanserin. The kilogram-scale synthesis began with the alkylation of commercially available 4-hydroxybenzaldehyde (123) with isobutyl bromide (124) under basic conditions. Condensation of the resultant benzaldehyde 125 with hydroxylamine furnished the corresponding oxime 126 in 63% yield from 123. Hydrogenation of 126 catalyzed by Pd/C under acidic conditions produced benzylamine 127, which was isolated as the acetate salt in 41% yield. This compound underwent sodium hydroxide workup followed by reaction with HCl gas and phosgene to deliver isocyanate 128. Nucleophilic attack of this isocyanate by benzylamine 129 (prepared from reductive amination of commercially available N-methylpiperid-4-one 130 with 4-fluorobenzylamine 131) followed by salt formation using tartaric acid in aqueous isopropyl acetate, and THF completed the synthesis of pimavanserin tartrate (XI) in 50% yield over the two-step protocol and a 10.6% overall yield from 123.
Properties of Unii-na83F1sjsr
| storage temp. | Store at -20°C |
| solubility | DMSO:70.0(Max Conc. mg/mL);69.6(Max Conc. mM) |
| form | Solid |
| color | White to off-white |
Safety information for Unii-na83F1sjsr
Computed Descriptors for Unii-na83F1sjsr
Unii-na83F1sjsr manufacturer
ALS India Life Sciences Pvt. Ltd
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![N-[(4-Fluorophenyl)methyl]-1-methyl-4-piperidinamine](https://img.chemicalbook.in/CAS/GIF/359878-47-0.gif)


You may like
-
 706782-28-7 Pimavanserin tartrate 98%View Details
706782-28-7 Pimavanserin tartrate 98%View Details
706782-28-7 -
 706782-28-7 98%View Details
706782-28-7 98%View Details
706782-28-7 -
 Pimavanserin tartrate 706782-28-7 98.33%View Details
Pimavanserin tartrate 706782-28-7 98.33%View Details
706782-28-7 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
