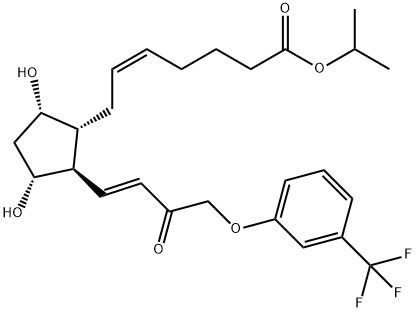
Travoprost
Synonym(s):(±)-16-(m-Trifluoromethylphenoxy)tetranorprostaglandin F2;Travoprost;Travoprost solution
- CAS NO.:157283-68-6
- Empirical Formula: C26H35F3O6
- Molecular Weight: 500.55
- MDL number: MFCD03411995
- EINECS: 682-028-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
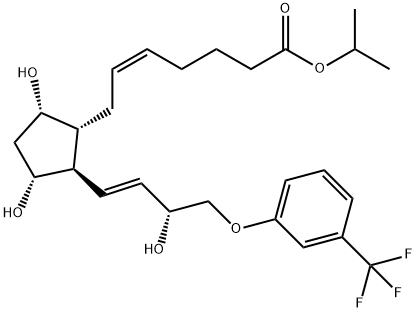
What is Travoprost?
Absorption
Travoprost is systemically absorbed through the cornea . In humans, peak plasma concentrations of travoprost free acid were low (25 pg/mL or less) and occurred within 30 minutes following topical ocular administration of one drop of 0.004% travoprost ophthalmic solution .
Toxicity
No cases of overdose have been reported for travoprost . A topical overdose is not likely to occur or to be associated with toxicity . A topical overdose of travoprost may be flushed from the eye(s) with lukewarm water . Treatment of a suspected oral ingestion is symptomatic and supportive .
Travoprost has harmful pharmacological effects on pregnancy and/or the fetus/new-born child. Travoprost should not be used during pregnancy unless clearly necessary . The medication subsequently must not be used in women of childbearing age/potential unless adequate contraceptive measures are in place .
It is unknown whether travoprost from the eye drops is excreted in human breast milk. Animal studies have shown excretion of travoprost and metabolites in breast milk . The use of travoprost by breast-feeding mothers is not recommended .
There are no data on the effects of TRAVATAN on human fertility . Animal studies showed no effect of travoprost on fertility at doses more than 250 times the maximum recommended human ocular dose .
Use in patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use .
No overall clinical differences in safety or effectiveness have been observed between elderly and other adult patients .
Travoprost has been studied in patients with mild to severe hepatic impairment and in patients with mild to severe renal impairment (creatinine clearance as low as 14 ml/min) . No dosage adjustment is necessary for these patients .
Description
Travoprost was launched in the US as Travatan?, an ophthalmic solution (0.004%) administered topically for the treatment of elevated intraocular hypertension (lOP) through open-angle glaucoma, a common optic neuropathy and a leading cause of blindness. Travoprost is the isopropyl ester of (+)-fluprostenol, a new prostaglandin derivative belonging to the PGF2α, analog class. This compound can be prepared in 8 steps from a bicyclic lactone aldehyde by a Wittig alkylidenation followed, after 2 ketonic reductions, by a lactol opening to prostenoic acid while protecting and deprotecting appropriately. Travoprost is a full agonist of FP receptors with a greater affinity than PGF2α, (K1 = 52 nM). Pharmacologic studies in rabbits treated daily for a week demonstrated a significant increase of the microvascular optic nerve head blood flow without significant alterations in the systemic flow. In several placebo-controlled clinical studies with hundreds of patients suffering from open-angle glaucoma or ocular hypertension, travoprost (1 to 4 pm daily) dose-dependently reduced lOP (by about 30% at 4 pm); it was shown equal or superior to latanoprost, another prostaglandin analog (5 pm daily) or the adrenergic ~-blocker timolol (500 pm bid). In addition, travoprost was safe and well tolerated with a low incidence of conjunctival hyperaemia.
Chemical properties
Colorless Oil
Originator
Alcon (US)
The Uses of Travoprost
Travoprost is a selective FP prostaglandin receptor agonist used to treat glaucoma (1,2).
The Uses of Travoprost
Selective FP prostaglandin receptor agonist. Isopropyl ester of (+)-fluprostenol. Antiglaucoma
The Uses of Travoprost
treatment conditions arising from excess uric acid, eg chronic gout
Background
Travoprost ophthalmic solution is a topical medication used for controlling the progression of glaucoma or ocular hypertension, by reducing intraocular pressure . It is a synthetic prostaglandin F2alpha analog . Having been a well-received therapeutic agent with demonstrated efficacy and safety, travoprost is currently approved by the US FDA as a first-line treatment for lowering intraocular pressure in patients with open-angle glaucoma or ocular hypotension . Furthermore, this approval also solidifies the medication as the first and only prostaglandin analog approved by the FDA for first-line treatment of glaucoma patients that does not contain the preservative benzalkonium chloride . Moreover, travoprost is also currently approved in the EU for the decrease of elevated intraocular pressure in paediatric patients aged 2 months to < 18 years with ocular hypertension or paediatric glaucoma .
What are the applications of Application
Fluprostenol isopropyl ester is a prodrug PGF2αR agonist
Indications
Travoprost ophthalmic solution is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension .
Travoprost is also currently indicated for the decrease of elevated intraocular pressure in paediatric patients aged 2 months to < 18 years with ocular hypertension or paediatric glaucoma .
What are the applications of Application
15(S)-Fluprostenol isopropyl ester is an unnatural C-15 epimer of Travoprost
Definition
ChEBI: The isopropyl ester of prostaglandin F2alpha in which the pentyl group is replaced by a 3-(trifluoromethyl)phenoxymethyl group. A synthetic analogue of prostaglandin F2alpha, ophth lmic solutions of travoprost are used as a topical medication for controlling the progression of open-angle glaucoma and ocular hypertension, by reducing intraocular pressure. It is a pro-drug; the isopropyl ester group is hydrolysed by esterases in the co nea to the biologically active free acid, fluprostenol.
brand name
Travatan
General Description
Travoprost (Travatan and Travatan-Z) is supplied asa 2.5- or 5.0-mL sterile 0.004% ophthalmic solution in a4.0- or 7.5-mL size container. Travoprost is claimed to bethe most potent and FP-specific analog in this product category. Cautions and side effects are similar to those givenpreviously.
Pharmacokinetics
Travoprost, an isopropyl ester prodrug, is a synthetic prostaglandin F2 alpha analog that is rapidly hydrolyzed by esterases in the cornea to its biologically active free acid . The travoprost free acid is potent and highly selective for the FP prostanoid receptor .
Metabolism
Travoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to its biologically active free acid . Systemically, travoprost free acid is rapidly and extensively metabolized in the kidney, liver, and lung to inactive metabolites via beta-oxidation of the α(carboxylic acid) chain to give the 1,2-dinor and 1,2,3,4-tetranor analogs, via oxidation of the 15-hydroxyl moiety, as well as via reduction of the 13,14 double bond .
Properties of Travoprost
| Boiling point: | 584.8±50.0 °C(Predicted) |
| alpha | D20 +14.6° (c = 1.0 in methylene chloride) |
| Density | 1.245±0.06 g/cm3(Predicted) |
| Flash point: | 17 °C |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Dichloromethane (Sparingly, Sonicated), Chloroform (Sparingy), Methanol (Sparing |
| pka | 13.43±0.20(Predicted) |
| form | ethanol solution |
| color | Colourless to Light Beige |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 157283-68-6(CAS DataBase Reference) |
Safety information for Travoprost
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Travoprost
| InChIKey | MKPLKVHSHYCHOC-AHTXBMBWSA-N |
Travoprost manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Travoprost 98%View Details
Travoprost 98%View Details
157283-68-6 -
 Travoprost 157283-68-6 98%View Details
Travoprost 157283-68-6 98%View Details
157283-68-6 -
 Travoprost 95.00% CAS 157283-68-6View Details
Travoprost 95.00% CAS 157283-68-6View Details
157283-68-6 -
 Fluprostenol isopropyl ester CAS 157283-68-6View Details
Fluprostenol isopropyl ester CAS 157283-68-6View Details
157283-68-6 -
 Travoprost CAS 157283-68-6View Details
Travoprost CAS 157283-68-6View Details
157283-68-6 -
 Travoprost CAS 157283-68-6View Details
Travoprost CAS 157283-68-6View Details
157283-68-6 -
 (5Z)-isopropyl 7-((1R,2R,3R,5S)-2-((R,E)-4-(3-(trifluoromethyl)phenoxy)-3-hydroxybut-1-enyl)-3,5-dihydroxycyclopentyl)hept-5-enoate; Travoprost 95.00%View Details
(5Z)-isopropyl 7-((1R,2R,3R,5S)-2-((R,E)-4-(3-(trifluoromethyl)phenoxy)-3-hydroxybut-1-enyl)-3,5-dihydroxycyclopentyl)hept-5-enoate; Travoprost 95.00%View Details
157283-68-6 -
 Travoprost API (API)View Details
Travoprost API (API)View Details
157283-68-6
