Titanium dioxide
Synonym(s):Titanium dioxide;Titanium;Titanium(IV) oxide;Titania;TiOxide
- CAS NO.:13463-67-7
- Empirical Formula: O2Ti
- Molecular Weight: 79.8658
- MDL number: MFCD00011269
- EINECS: 236-675-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:22
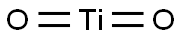
What is Titanium dioxide?
Absorption
When male and female rats were fed a diet containing titanium dioxide (100 g/kg) for a period of about 32 days, a significant retention of titanium of 0.06 and 0.11 mg/kg wet weight was found only in the muscles; no retention was observed in the liver, spleen, kidney, bone, plasma, or erythrocytes
Toxicity
Titanium dioxide has been shown to be carcinogenic to animals, rat - LD50 Intratracheal (>100ug/kg) Effects: Structural or functional changes in the bronchi and trachea. However, there is insufficient evidence that titanium dioxide is carcinogenic to humans. Although it is classified as possibly carcinogenic to humans (Group 2B), titanium dioxide is generally considered safe by the FDA.
Description
Titanium dioxide is a white powder with the highest hiding power among all white pigments. Although it is noncombustible, it can cause a dust explosion if suspended in air and exposed to an ignition source. However, it is not classified as hazardous by the DOT for transportation. Its main uses include serving as a white pigment in paints, paper, rubber, and plastics, and in sunscreens to protect skin from ultraviolet rays.
Chemical properties
Titanium dioxide is a white, amorphous, odorless, and tasteless nonhygroscopic powder. While its average particle size is less than 1 mm, commercial forms typically aggregate into particles around 100 mm in diameter. It can exist in several crystalline forms—rutile, anatase, and brookite—but only rutile and anatase are commercially important. Rutile is more thermodynamically stable, whereas anatase is more commonly used in pharmaceutical applications.
Physical properties
Anatase is metastable over long periods despite being less thermodynamically stable than rutile. However, it irreversibly and rapidly converts to rutile above 700°C. Anatase is more transparent in the near-UV than rutile, with an absorption edge at 385 nm. It absorbs less blue light and has a blue tone.
The Uses of Titanium dioxide
Titanium dioxide (TiO?) is one of the 21 FDA-approved sunscreen chemicals, with an approved usage level of 2 to 25 percent. It stays on the skin’s surface to scatter UV light and is often combined with other sunscreen chemicals to enhance SPF value, reducing the risk of irritation or allergies from excessive chemical use. Its use in sunscreens, makeup bases, and daytime moisturizers depends on particle size: smaller particles are less visible, while larger ones can leave a whitish cast. Some companies label particles as “micro” or “ultra” based on size. Given its chemical, cosmetic, and physical properties, titanium dioxide may be an ideal UVA/UVB protection component and is also used for providing white color in cosmetics.
Background
Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium. It is used as a pigment under the names titanium white, Pigment White 6 (PW6), or CI 77891. It is typically extracted from ilmenite, rutile and anatase.
Indications
Titanium dioxide is used in most sunscreens to block UVA and UVB rays, similar to zinc oxide.
What are the applications of Application
Titanium(IV) oxide is a bacteriocide commonly used in paint and cosmetics
Production Methods
Titanium dioxide naturally exists as the minerals rutile (with a tetragonal structure), anatase (also tetragonal), and brookite (orthorhombic). Commercially, it can be produced via the sulfate or chloride process. The sulfate process involves digesting a titanium-bearing ore like ilmenite in sulfuric acid, dissolving the sulfates in water, precipitating hydrous titanium dioxide through hydrolysis, and then calcining the product at high temperatures. In the chloride process, dry ore is chlorinated at high temperatures to form titanium tetrachloride, which is then oxidized to produce titanium dioxide.
Safety Profile
Titanium dioxide is considered a nuisance dust and can irritate human skin. It is also classified as a questionable carcinogen, with experimental evidence suggesting potential carcinogenic, neoplastigenic, and tumorigenic effects. Additionally, it can react violently or incandescently with certain metals (such as aluminum, calcium, magnesium, potassium, sodium, zinc, and lithium) at high temperatures.
Properties of Titanium dioxide
| Melting point: | 1840 °C |
| Boiling point: | 2900 °C |
| Density | 4.26 g/mL at 25 °C(lit.) |
| refractive index | 2.61 |
| Flash point: | 2500-3000°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Practically insoluble in water. It does not dissolve in dilute mineral acids but dissolves slowly in hot concentrated sulfuric acid. |
| form | powder |
| color | White to slightly yellow |
| Specific Gravity | 4.26 |
| Odor | at 100.00?%. odorless |
| PH | 7-8 (100g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Crystal Structure | Orthorhombic, Pcab |
| Merck | 14,9472 |
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 5000 mg/m3; TWA 2.4 mg/m3; TWA 0.3 mg/m3 |
| Dielectric constant | 2.9(20℃) |
| CAS DataBase Reference | 13463-67-7(CAS DataBase Reference) |
| IARC | 2B (Vol. 47, 93) 2010 |
| NIST Chemistry Reference | Titanium dioxide(13463-67-7) |
| EPA Substance Registry System | Titanium dioxide (13463-67-7) |
Safety information for Titanium dioxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Titanium dioxide
Titanium dioxide manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 TITANIUM DIOXIDE 99%View Details
TITANIUM DIOXIDE 99%View Details -
 Titanium Dioxide 99%View Details
Titanium Dioxide 99%View Details -
 Titanium Dioxide (Anatase) 99%View Details
Titanium Dioxide (Anatase) 99%View Details -
 Titanium Di Oxide 99%View Details
Titanium Di Oxide 99%View Details -
 Dupont R902 Ti Pure Titanium Dioxide, PowderView Details
Dupont R902 Ti Pure Titanium Dioxide, PowderView Details
1317-80-2 -
 White/Off-White Titanium Dioxide PowderView Details
White/Off-White Titanium Dioxide PowderView Details
1317-70-0 -
 Titanium Dioxide, 25 kg, CAS Number: 1317-70-0View Details
Titanium Dioxide, 25 kg, CAS Number: 1317-70-0View Details
1317-70-0 -
 Titanium Dioxide Anatase Powder, CAS Number: 1317-70-0View Details
Titanium Dioxide Anatase Powder, CAS Number: 1317-70-0View Details
1317-70-0
