Sodium nitrite
Synonym(s):Sodium Nitrite;Sodium nitrite solution;Anti-Rust;Diazotizing salts;Nitrite standard for aqueous matrices ready foruse
- CAS NO.:7632-00-0
- Empirical Formula: NaNO2
- Molecular Weight: 69
- MDL number: MFCD00011118
- EINECS: 231-555-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:23:41

What is Sodium nitrite?
The Uses of Sodium nitrite

To ACN (350 mL) at RT was added CuBr2 (67 g, 300 mmol). The mixture was cooled to 0-5 C and was treated dropwise with t-BuONO (72.5 mL, 535 mmol). The mixture was stirred for 5 min, then was added the SM (35 g, 238 mmol). The reaction was stirred at RT for 2 h. EtOAc (525 mL) was added to the mixture and the reaction was quenched with 10% aq NH4Cl (350 mL) and 2% aq NH3 (175 mL). The layers were separated and the aq layer was extracted with EtOAc. The combined organics were dried and concentrated. The resulting material was purified by silica gel chromatography (eluting with 5% EtOAc/hexane) to provide the product as a yellow solid. [35 g, 70%]
Definition
Sodium nitrite is an inorganic sodium salt having nitrite as the counterion. It can be used as a food preservative and antidote to cyanide poisoning. It is a nitrite salt and an inorganic sodium salt.
The Uses of Sodium nitrite
Sodium nitrite is used in heat transfersalts, metal treatment and finishing, as a color fixative andpreservative for meats and fish, in pharmaceuticals, and as anantidote for Cyanide poisoning.
Toxicity
Sodium nitrite is toxic. The LD50 in rats is 180 mg/kg and in humans LDLo is 71 mg/kg.
Environmental Fate
Partition Behavior in Water, Sediment, and Soil
This substance dissociates immediately into sodium and nitrite ions in water. Concentrations of nitrate in rainwater of up to 5mg/L have been observed in industrial areas.
Safety
Sodium nitrite is a yellowish white crystalline solid. Noncombustible but will accelerate the burning of combustible material. If large quantities are involved in a fire or if the combustible material is finely divided, an explosion may result. If contaminated by ammonium compounds, spontaneous decomposition can occur and the resulting heat may ignite surrounding combustible material. Prolonged exposure heat may result in an explosion.Toxic oxides of nitrogen are produced in fires involving Sodium nitrite.
Medical Uses
Sodium nitrite is the most prevalent drug for cyanide poisoning. It takes approximately 12 min to generate approximately 40% of methemoglobin after intravenous administration of the recommended dose. Despite this delay in inducing a significant level of methemoglobinemia, reasonable protection offered by sodium nitrite can be attributed to its vasodilatory effects.
Properties of Sodium nitrite
| Melting point: | 271 °C (lit.) |
| Boiling point: | 320 °C |
| Density | 2.17g/cm3 |
| storage temp. | 2-8°C |
| solubility | aqueous acid: 1 - 2μl acetic acid per ml H2Osoluble |
| form | powder |
| appearance | White to slightly yellow solid |
| Specific Gravity | 2.168 |
| color | White or colorless |
| PH Range | 9 |
| PH | 9 (100g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | 820 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8648 |
| Stability: | Stable. Incompatible with reducing agents, strong oxidizing agents, organics and other flammable materials, finely powdered metals. Contact with combustible material may lead to fire. Hygroscopic. |
| CAS DataBase Reference | 7632-00-0(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium nitrite (7632-00-0) |
Safety information for Sodium nitrite
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H319:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium nitrite
| InChIKey | LPXPTNMVRIOKMN-UHFFFAOYSA-M |
Sodium nitrite manufacturer
JSK Chemicals
New Products
4-Iodo-3,5-dimethylbenzonitrile 2-fluoro-4-iodoaniline 2-(3-Methyl-1,2,4-oxadiazol-5-yl)acetic acid (as potassium salt) 6,6'-methylenebis(9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-2,3-dihydro-1H-carbazol-4(9H)-one) 3-Bromo-2-fluorobenzoic acid Quinuclidine-4-carbonitrile N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 1,4-bis(methylsulfonyl)butane 4-Cyano-N-Methacryloyl-3-Trifluoromethyl Aniline 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzeneRelated products of tetrahydrofuran
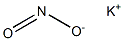
You may like
-
 sodiumnitrite 98%View Details
sodiumnitrite 98%View Details -
 Sodium Nitrite 99%View Details
Sodium Nitrite 99%View Details -
 Sodium Nitrite 99%View Details
Sodium Nitrite 99%View Details -
 Sodium nitrite 99%View Details
Sodium nitrite 99%View Details -
 Sodium nitrite 98%View Details
Sodium nitrite 98%View Details -
 Sodium nitrite 98%View Details
Sodium nitrite 98%View Details -
 Sodium Nitrite CASView Details
Sodium Nitrite CASView Details -
 Nitrite standard solution CASView Details
Nitrite standard solution CASView Details
