SB 290157 trifluoroacetate salt
Synonym(s):N²-[(2,2-Diphenylethoxy)acetyl]-L-arginine, TFA;N2-[2-(2,2-Diphenylethoxy)acetyl]-L-arginine trifluoroacetate salt;SB 290157 - CAS 1140525-25-2 - Calbiochem;SB 290157 trifluoroacetate salt
- CAS NO.:1140525-25-2
- Empirical Formula: C24H29F3N4O6
- Molecular Weight: 526.51
- MDL number: MFCD04974198
- Update Date: 2026-02-02 18:10:39
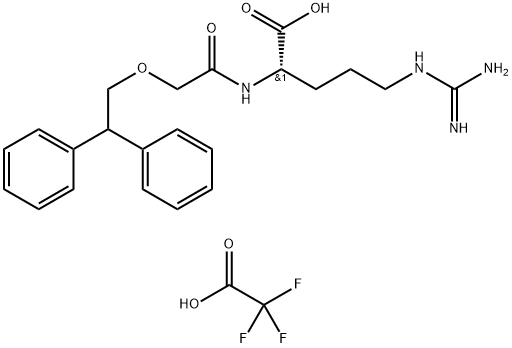
What is SB 290157 trifluoroacetate salt?
The Uses of SB 290157 trifluoroacetate salt
SB 290157 Trifluoroacetate Salt is a selective, high affinity, competitive antagonist of anaphylatoxin C3a receptor.
What are the applications of Application
SB 290157 trifluoroacetate salt is a selective, high affinity, competitive antagonist of anaphylatoxin C3a receptor
General Description
A non-peptide with anti-inflammatory properties that acts as a selective, high affinity, competitive antagonist of the anaphylatoxin C3a receptor (C3aR; IC50 = 200 nM). Does not antagonize C5aR or other chemotactic G-protein coupled receptors. Blocks C3a-induced internalization of C3aR in human neutrophils and C3a-induced Ca2+ mobilization in basophilic leukemia RBL-2H3 cells expressing murine, guinea pig, or human C3aR (IC50 = 7, 12.5, and 27.7 nM, respectively). Also blocks C3a-mediated ATP release from guinea pig platelets (IC50 = 30 nM).
Biological Activity
sb 290157 is a c3ar antagonist.the anaphylatoxin c3a, a potent chemotactic peptide and inflammatory mediator, binds to and activates a g-protein-coupled receptor. molecular cloning of the c3ar has facilitated the identification of the non-peptide c3ar antagonists.
Biochem/physiol Actions
Target IC50: 200 nM against the anaphylatoxin C3a receptor; 7, 12.5, and 27.7 nM, in blocking C3a-induced internalization of C3aR in human neutrophils and C3a-induced Ca2+ mobilization in basophilic leukemia RBL-2H3 cells expressing murine, guinea pig, or human C3aR
in vitro
sb 290157 was a competitive antagonist of 125i-c3a and binding to rat basophilic leukemia (rbl)-2h3 cells expressing the human c3ar , with an ic50 of 200 nm. sb 290157 could also block the c3a-induced c3ar internalization concentration-dependently and c3a-induced ca21 mobilization in rbl-c3ar cells and human neutrophils. sb 290157 was founf to be selective for the c3ar in that it did not antagonize the c5ar or six other chemotactic g protein-coupled receptors. in addition, sb 290157 could also inhibit c3a induced ca21 mobilization of rbl-2h3 cells expressing the mouse and guinea pig c3ars [1].
in vivo
in animal models, sb 290157 was able to inhibit neutrophil recruitment in a guinea pig lps-induced airway neutrophilia model and decrease paw edema in a rat adjuvant-induced arthritis model [1].
Storage
Desiccate at RT
References
[1] r. s. ames, d. lee, j. j. foley, et al. identification of a selective nonpeptide antagonist of the anaphylatoxin c3a receptor that demonstrates antiinflammatory activity in animal models. journal of immunology 166(10), 6341-6348 (2001).
Properties of SB 290157 trifluoroacetate salt
| Melting point: | >43°C (dec.) |
| storage temp. | -20°C |
| solubility | Ethanol (Slightly, Sonicated), Methanol (Slightly), Water (Slightly) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +1.5 to +5°, c = 1.0 in DMSO |
| Stability: | Hygroscopic |
Safety information for SB 290157 trifluoroacetate salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SB 290157 trifluoroacetate salt
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 SB 290157 CAS 1140525-25-2View Details
SB 290157 CAS 1140525-25-2View Details
1140525-25-2 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
