S-Ruxolitinib (INCB018424)
- CAS NO.:941685-37-6
- Empirical Formula: C17H18N6
- Molecular Weight: 306.37
- MDL number: MFCD13184797
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
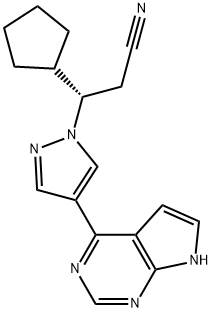
What is S-Ruxolitinib (INCB018424)?
The Uses of S-Ruxolitinib (INCB018424)
(3S)-3-Cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile is a newly developed JAK2 inhibitor therapy aimed to improve MPN-associated splenomegaly and systemic symptoms.
The Uses of S-Ruxolitinib (INCB018424)
(3S)-3-Cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile is a newly developed JAK2 inhibitor therapy aimed to improve MPN-associated splenomegaly and systemic symptoms. The opposite enantiomer of Ruxolitinib (R702000).
Biological Activity
s-ruxolitinib is the chirality of incb018424, is a potent and selective small-molecule janus kinase 1 (jak1) and jak2 inhibitor. it was initially developed to target the constitutive activation of the jak-stat pathway. janus kinases (jaks) are a family of cytoplasmic tyrosine kinases that mediates signals from the receptors for various cytokines and growth factors that have a key role in haematopoiesis and immune function. ruxolitinib maintains its anti-jak activity by competitive inhibition of the atp-binding catalytic site of the kinase domain. ruxolitinib is well absorbed at >95%. exposure of jak2v617f-positive ba/f3 cells to ruxolitinib iss shown to result in reduced cellular proliferation.ruben a. mesa, uma yasothan, peter kirkpatrick. ruxolitinib. nature reviews drug discovery. 2012; 11: 103-104john mascarenhas, ronald hoffman. ruxolitinib: the first fda approved therapy for the treatment of myelofibrosis. clinical cancer research. 2012; 18(11): 3008 - 3014
Properties of S-Ruxolitinib (INCB018424)
| Melting point: | 138-143oC |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.63±0.50(Predicted) |
| color | White |
Safety information for S-Ruxolitinib (INCB018424)
Computed Descriptors for S-Ruxolitinib (INCB018424)
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran


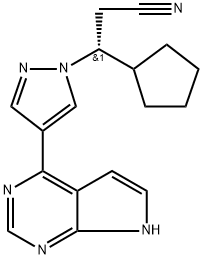
![3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile](https://img.chemicalbook.in/CAS/20180601/GIF/941688-05-7.gif)
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
