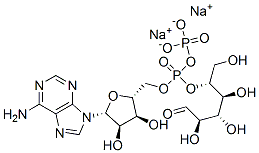
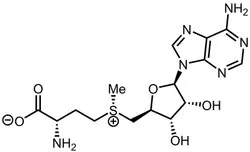
S-Adenosyl-L-methionine
- CAS NO.:29908-03-0
- Empirical Formula: C15H22N6O5S
- Molecular Weight: 398.44
- MDL number: MFCD00871208
- EINECS: 249-946-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-23 16:19:27
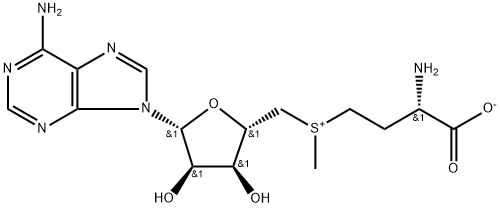
What is S-Adenosyl-L-methionine?
Absorption
S-Adenosylmethionine is absorbed from the small intestine following oral intake. As absorption is affected by food, it is best to take on an empty stomach. Bioavailability is low following oral intake.
Toxicity
Irritating to mucus membranes and upper respiratory tract. Can cause CNS depression.
Originator
Donamet ,Ravizza
Occurrence
SAM-e is found in all living cells and is a precursor in some amino acids.
The Uses of S-Adenosyl-L-methionine
white powder LOD 0.2%
Indications
S-Adenosylmethionine (SAMe) is used as a drug in Europe for the treatment of depression, liver disorders, fibromyalgia, and osteoarthritis. It has also been introduced into the United States market as a dietary supplement for the support of bone and joint health, as well as mood and emotional well being.
Background
Physiologic methyl radical donor involved in enzymatic transmethylation reactions and present in all living organisms. It possesses anti-inflammatory activity and has been used in treatment of chronic liver disease. (From Merck, 11th ed)
What are the applications of Application
Ademetionine is a common co-substrate involved in methyl group transfers to nucleic acids, proteins and lipids.
Definition
ChEBI: S-adenosyl-L-methioninate is a sulfonium betaine that is a conjugate base of S-adenosyl-L-methionine obtained by the deprotonation of the carboxy group. It has a role as a human metabolite. It is functionally related to a L-methioninate. It is a conjugate base of a S-adenosyl-L-methionine.
Manufacturing Process
S-Adenosyl methionine (SAM) is produced through the fermentation of Saccharomyces cerevisiae. The process involves several key stages:
Inoculum Preparation: Multiple strains of S. cerevisiae (IFO 2342, 2343, 2345, 2346, 2347) are individually cultivated in a seed medium at 28°C for 24 hours.
Fermentation: The seed culture is transferred to a larger fermentor containing a production medium rich in sucrose and L-methionine. The cultivation proceeds for 72 hours at 28°C with controlled aeration and agitation.
Extraction: After fermentation, the yeast cells are harvested by centrifugation. SAM is extracted from the cells using perchloric acid, followed by neutralization and removal of the resulting potassium perchlorate precipitate.
Purification and Isolation: The crude extract is purified using ion-exchange chromatography. SAM is first adsorbed onto a weakly acidic cation exchange resin (Amberlite IRC-50) and washed to remove impurities. It is then eluted with dilute sulfuric acid. The eluate's pH is adjusted, and the solution is lyophilized to yield SAM as a stable sulfate salt. This salt can be converted to the free base form if required. Analysis and Yield: The purity of the final product is confirmed using thin-layer chromatography (TLC), paper chromatography, and high-performance liquid chromatography (HPLC). The yields of SAM, based on the dry cell weight, were highest for strains IFO 2346 (18.8%), IFO 2347 (16.7%), and IFO 2343 (12.1%).
Therapeutic Function
Metabolic, Antiinflammatory
Benefits
S-Adenosyl-L-methionine (SAMe) is a naturally occurring compound involved in key metabolic pathways. It contributes to immune function, the maintenance of cell membranes, and the synthesis and metabolism of several key neurotransmitters, including serotonin, melatonin, and dopamine. The biological activity of SAMe is interdependent with vitamin B12 and folate (vitamin B9), and a deficiency in either of these vitamins can lead to reduced levels of SAMe. Clinical studies suggest that SAMe may be effective in managing certain health conditions. Research indicates that oral administration of SAMe can alleviate the pain associated with osteoarthritis, with an efficacy comparable to nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen. Furthermore, other studies suggest a potential role for SAMe as a therapeutic agent in the treatment of depression.
Pharmacokinetics
S-adenosylmethionine is an intermediate metabolite of methionine. Its involvement in methylation assists in cellular growth and repair, maintains the phospho-bilipid layer in cell membranes. It also helps in the maintenance of the action of several hormones and neurotransmitters that affect mood. Highest concentration are found in the brain and the liver.
Metabolism
Significant first-pass metabolism in the liver. Approximately 50% of S-Adenosylmethionine (SAMe) is metabolized in the liver. SAMe is metabolized to S-adenosylhomocysteine, which is then metabolized to homocysteine. Homocysteine can either be metabolized to cystathionine and then cysteine or to methionine. The cofactor in the metabolism of homocysteine to cysteine is vitamin B6. Cofactors for the metabolism of homocysteine to methionine are folic acid, vitamin B12 and betaine.
Properties of S-Adenosyl-L-methionine
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Sonicated), Water (Slightly) |
| form | Solid |
| color | White to Yellow |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 29908-03-0(CAS DataBase Reference) |
Safety information for S-Adenosyl-L-methionine
Computed Descriptors for S-Adenosyl-L-methionine
| InChIKey | MEFKEPWMEQBLKI-WGIZDKKNNA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](C[S+](C)CC[C@H](N)C(=O)[O-])O[C@H]1N1C=NC2C(=NC=NC1=2)N)O |&1:1,2,3,9,15,r| |
S-Adenosyl-L-methionine manufacturer
Innovative
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 29908-03-0 S-(5'-Adenosyl)L-methionine 98%View Details
29908-03-0 S-(5'-Adenosyl)L-methionine 98%View Details
29908-03-0 -
 S-Adenosyl-L-methionine, 99% CAS 29908-03-0View Details
S-Adenosyl-L-methionine, 99% CAS 29908-03-0View Details
29908-03-0 -
 Ademetionine 97% CAS 29908-03-0View Details
Ademetionine 97% CAS 29908-03-0View Details
29908-03-0 -
 S-Adenosyl-L-Methionine, Purity: Greater Than 99%, 1 kg / 5 kg / 10 kg / 25 kgView Details
S-Adenosyl-L-Methionine, Purity: Greater Than 99%, 1 kg / 5 kg / 10 kg / 25 kgView Details
29908-03-0 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
