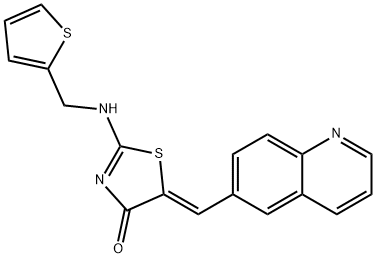
Ro 3306
Synonym(s):(5Z)-5-Quinolin-6-ylmethylene-2-[(thiophen-2-ylmethyl)-amino]-thiazol-4-one;5-(6-Quinolinylmethylene)-2-[(2-thienylmethyl)amino]-4(5H)-thiazolone
- CAS NO.:872573-93-8
- Empirical Formula: C18H13N3OS2
- Molecular Weight: 351.45
- MDL number: MFCD17392573
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
What is Ro 3306?
Description
RO-3306 (872573-93-8) is a selective inhibitor of CDK1 (IC50?= 35 nM versus CDK2 IC50?= 340nM).1,2?It induces G2/M phase cell cycle arrest and apoptosis. Inhibition of CDK1 with RO-3306 has been shown to have synergistic effects with PARP inhibitors in treating various breast cancers.3,4?It has also been demonstrated to overcome apoptotic resistance in BRAFV600E?human colorectal cancer cells.5
The Uses of Ro 3306
RO-3306 is an ATP-competitive and selective CDK1 inhibitor used against a diverse panel of human kinases. Used in the treatment of malignant tumors in conjunction with PARP inhibitors.
The Uses of Ro 3306
RO-3306 has been used:
- To study the significance of CA4-mediated cytotoxicity in mitotic arrest
- In cell cycle synchronization to conduct a study on proteomics
- As a CDK1 inhibitor, to prevent early mitotic entry
What are the applications of Application
RO-3306 is an inhibitor of CDK1/cyclin B1 and CDK1/cyclin A
Biochem/physiol Actions
RO-3306 is a selective ATP-competitive inhibitor of CDK1. It inhibites CDK1 cyclin B1 activity with Ki of 35 nM, nearly 10-fold selectivity relative to CDK2/cyclin E and over 50-fold relative to CDK4/cyclin D. RO-3306 has been used to cause cell cycle arrest at the G2/M boundary.
Storage
Store at +4°C
References
1) Vassilev?et al.?(2006),?Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1; Proc. Nat. Acad. Sci. USA?103?10660 2) Krasinska?et al.?(2008),?Selective chemical inhibition as a tool to study Cdk1 and Cdk2 functions in the cell cycle; Cell Cycle?7?1702 3) Pierce?et al.?(2013),?Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines; Cancer Biol. Ther.?14?537 4) Xia?et al.?(2014),?The CDK1 inhibitor RO3306 improves the response of BRCA-proficient breast cancer cells to PARP inhibition;?Int. J. Oncol.?44?735 5) Zhang?et al.?(2018),?Targeting CDK1 and MEK/ERK Overcomes Apoptotic Resistance in BRAF-Mutant Human Colorectal Cancer; Mol. Cancer Res.?16?378
Properties of Ro 3306
| Boiling point: | 569.1±60.0 °C(Predicted) |
| Density | 1.41 |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL, clear |
| pka | 3.99±0.10(Predicted) |
| form | powder |
| color | white to light brown |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Ro 3306
Computed Descriptors for Ro 3306
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 RO-3306 CAS 872573-93-8View Details
RO-3306 CAS 872573-93-8View Details
872573-93-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8