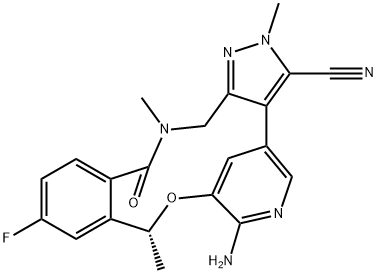
Ripretinib
- CAS NO.:1442472-39-0
- Empirical Formula: C24H21BrFN5O2
- Molecular Weight: 510.36
- MDL number: MFCD31657437
- Update Date: 2026-01-27 08:46:37
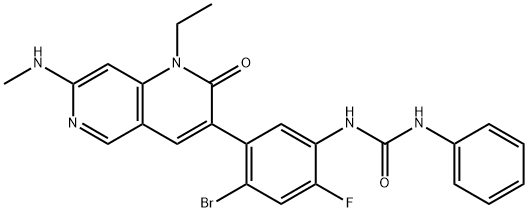
What is Ripretinib?
Absorption
Ripretinib is absorbed in the gastrointestinal tract and Tmax is achieved in 4 hours, with steady-state concentrations reached within 14 days.
Toxicity
There is limited information in the literature regarding an overdose with ripretinib, and LD50 information is not readily available. Like other kinase inhibitors, an overdose with ripretinib likely results in hematological toxicity, skin toxicity, as well as muscle, liver, and gastrointestinal toxicity.
The Uses of Ripretinib
Ripretinib is used in the treatment of gastrointestinal stromal tumors (GISTs).
Indications
Ripretinib is indicated to treat adults diagnosed with advanced gastrointestinal stromal tumor (GIST) who have had prior therapy with at least 3 kinase inhibitors, including with imatinib.
Background
Ripretinib is a kinase inhibitor used for the treatment of advanced gastrointestinal stromal tumor (GIST) that has not adequately responded to other kinase inhibitors such as sunitinib and imatinib. Ripretinib, also known as Qinlock, is manufactured by Deciphera Pharmaceuticals and was initially approved by the FDA on May 15, 2020.
It is the first drug approved as a fourth-line therapy in the specific setting of prior treatment with a minimum of 3 other kinase inhibitors.
brand name
Qinlock
General Description
Class: receptor tyrosine kinase; Treatment: GIST; Other name: DCC-2618; Elimination half-life = 15 h; Protein binding = 98.8%
Biological Activity
Ripretinib is a highly effective inhibitor of both wild-type KIT and PDGFRA, with IC50 values around 3 nM. It also targets a wide range of KIT and PDGFRA mutants in GIST. Additionally, it inhibits various tumor-driving kinases such as PDGFRβ, TIE2, VEGFR2, and BRAF.
Pharmacokinetics
As a broad-spectrum kinase inhibitor, ripretinib inhibits various gene mutations, increasing progression-free survival in patients with advanced gastrointestinal stromal tumors (GIST). It is effective in treating mutations that are resistant to chemotherapy with other kinase inhibitors, such as imatinib.
Ripretinib has the propensity to cause cardiac dysfunction and new primary cutaneous malignancy. It is important to measure cardiac ejection fraction before and during treatment as well as to perform regular dermatological assessments.
Metabolism
Ripretinib is metabolized by the CYP3A subfamily of enzymes with contributions from CYP2D6 and CYP2E1 to its active metabolite, DP-5439.
Properties of Ripretinib
| Boiling point: | 568.6±50.0 °C(Predicted) |
| Density | 1.544±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:25.0(Max Conc. mg/mL);49.0(Max Conc. mM) |
| form | A solid |
| pka | 12.15±0.70(Predicted) |
| color | White to light yellow |
Safety information for Ripretinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ripretinib
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 1442472-39-0 Ripretinib 98%View Details
1442472-39-0 Ripretinib 98%View Details
1442472-39-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
