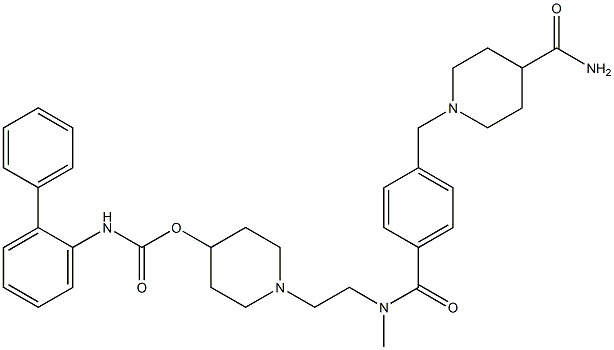
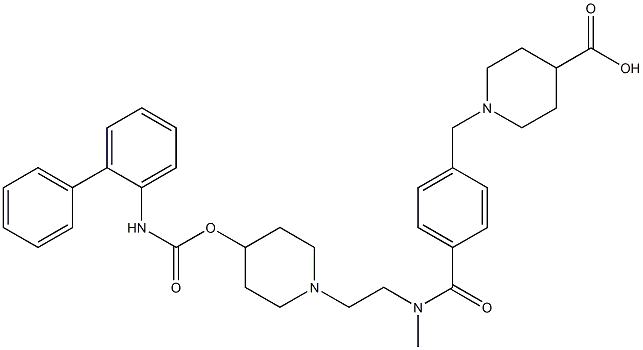
Revefenacin
- CAS NO.:864750-70-9
- Empirical Formula: C35H43N5O4
- Molecular Weight: 597.75
- MDL number: MFCD28386316
- Update Date: 2026-01-23 16:19:27
What is Revefenacin?
Absorption
In pharmacokinetic studies, revefenacin was absorbed very rapidly and presented a linear increase in plasma exposure with Cmax, tmax and AUC that ranged between 0.02-0.15 ng/ml, 0.48-0.51 hours and 0.03-0.36 ng.h/ml, respectively. The bioaccumulation of revefenacin was very limited and the steady-state was achieved by day 7.
Toxicity
Revefenacin does not produce the typical systemic effects associated with anticholinergic therapies. In carcinogenic studies in animals, there was no evidence of tumorigenicity. As well, there was no evidence of mutagenicity in the Ames test nor genotoxicity in in vitro mouse lymphoma assays and in vivo rat bone marrow micronucleus assays. There is no effect in the fertility.
In overdose situations, the common signs and symptoms are nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure, obstipation and difficulties in voiding.
Description
Revefenacin is a synthetic anticholinergic that is prescribed for the treatment of chronic obstructive pulmonary disease (COPD).
The Uses of Revefenacin
Revefenacin is used to treat chronic obstructive pulmonary disease (COPD), a long-term (chronic) lung disease that includes chronic bronchitis, emphysema, or both. It is an anticholinergic drug that helps the muscles around the airways in the lungs stay relaxed to prevent symptoms such as wheezing, coughing, chest tightness, and shortness of breath.
Indications
Revefenacin is indicated as an inhalation solution for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
COPD is a growing disease being the third leading cause of death in the US. This disease is characterized by not fully reversible airflow limitation.
Background
Revefenacin is a novel biphenyl carbamate tertiary amine agent that belongs to the family of the long-acting muscarinic antagonists (LAMA). The labile primary amide in the structure produces a "soft-drug" site that allows rapid systemic clearance and minimizing of the systemically mediated adverse reactions. The LAMA group falls into a parent category known as long-acting inhaled bronchodilators and this type of agents are recommended as a maintenance therapy for chronic obstructive pulmonary disease (COPD). From the LAMA group, revefenacin is the first once-daily nebulized LAMA treatment. It was developed by Theravance Biopharma and FDA approved on November 9, 2018.
What are the applications of Application
Revefenacin is an FDA-approved anticholinergic drug, a new tertiary amine biphenylcarbamate agent that also belongs to the long-acting muscarinic antagonist (LABA) family. It has the effect of relaxing the muscles around the airways of the lungs to make breathing easier. The structurally unstable tertiary amide produces a "soft drug" site, allowing rapid systemic clearance and minimising systemically mediated adverse effects. It can be used to treat symptoms such as wheezing, shortness of breath, cough and chest tightness in patients with chronic obstructive pulmonary disease (COPD: a group of diseases affecting the lungs and airways, including chronic bronchitis and emphysema).
brand name
Yupelri
Pharmacokinetics
Revefenacin has been reported to produce a sustained, long-acting bronchodilation with lower anti-muscarinic-related side effects. In clinical trials, revefenacin demonstrated to be of a long duration of action and low systemic exposure in patients with COPD. Also, it was reported that a dose of 88 mcg can produce a clinically effective bronchodilation measured by through forced expiratory volume in 1s and serial spirometric assessments.
In placebo-controlled trials, revefenacin showed a decrease in the use of albuterol rescue inhalers and sustained increases in the peak expiratory flow rate that reached a steady state at a maximum in day 7. As well, there was a reported superior lung selectivity index when compared with other LAMAs such as glycopyrronium and tiotropium which produced a decreased sialagogue effect.
Side Effects
Common adverse reactions in clinical trials (1%-10%) were headache, nasopharyngitis, upper respiratory tract infection, back pain, bronchitis, dizziness, and hypertension.
Metabolism
Revefenacin presents a high metabolic liability producing a rapid metabolic turnover after being distributed from the lung. This metabolic process is done primarily via enzymatic hydrolysis via CYP2D6 to its major hydrolytic metabolite THRX-195518.
Properties of Revefenacin
| Boiling point: | 777.5±60.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : ≥ 125 mg/mL (209.12 mM) |
| form | Solid |
| pka | 13.34±0.70(Predicted) |
| color | White to off-white |
Safety information for Revefenacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Revefenacin
| InChIKey | FYDWDCIFZSGNBU-UHFFFAOYSA-N |
| SMILES | C(OC1CCN(CCN(C(=O)C2=CC=C(CN3CCC(C(N)=O)CC3)C=C2)C)CC1)(=O)NC1=CC=CC=C1C1=CC=CC=C1 |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

![Carbamic acid, N-[1,1'-biphenyl]-2-yl-, 1-methyl-4-piperidinyl ester](https://img.chemicalbook.in/CAS/20200611/GIF/1289178-26-2.gif)
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](https://img.chemicalbook.in/CAS/20180703/GIF/743460-48-2.gif)

You may like
-
 864750-70-9 Revefenacin 98%View Details
864750-70-9 Revefenacin 98%View Details
864750-70-9 -
 Revefenacin 864750-70-9 98%View Details
Revefenacin 864750-70-9 98%View Details
864750-70-9 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
